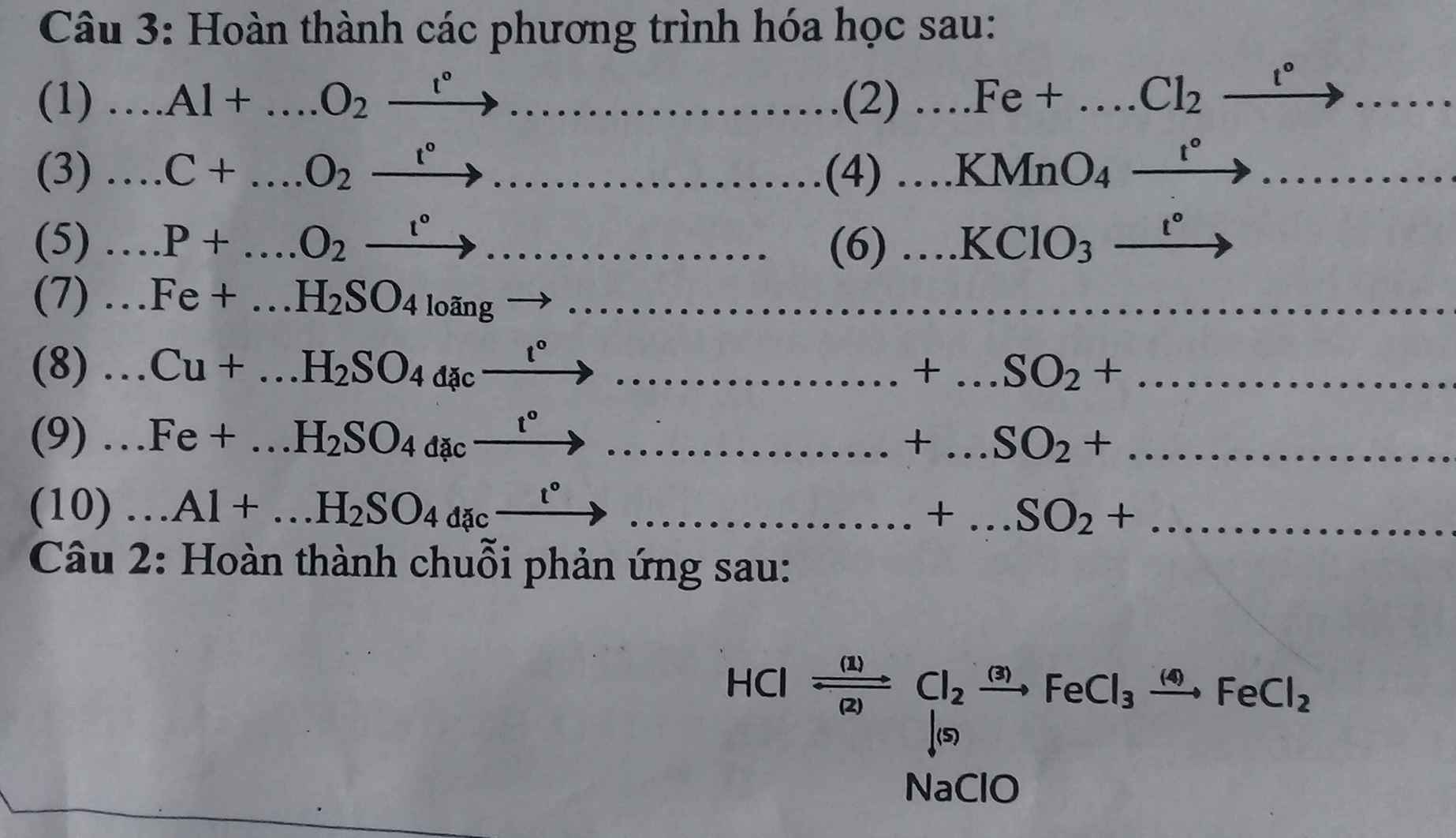
Gọi số mol Al, Cu, Fe là a, b , c
=> 27a + 64b + 56c = 17,5
\(n_{H_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\)
PTHH: 2Al + 6HCl --> 2AlCl3 + 3H2
______a------------------------>1,5a
Fe + 2HCl --> FeCl2 + H2
c------------------------>c
=>1,5a + c = 0,3
PTHH: 2Al + 3Cl2 --to--> 2AlCl3
_____a------------------>a
2Fe + 3Cl2 --to--> 2FeCl3
c------------------->c
Cu + Cl2 --to--> CuCl2
b---------------->b
=> 133,5a + 135b + 162,5c = 51,225
=> a = 0,1; b = 0,1; c = 0,15
=> \(\%Fe=\dfrac{56.0,15}{17,5}.100\%=48\%\)
=> A