Câu 4:
a) CTPT: CnH2n+2
\(n_{CO_2}=\dfrac{5,6}{22,4}=0,25\left(mol\right)\)
=> \(n_{C_nH_{2n+2}}=\dfrac{0,25}{n}\left(mol\right)\)
=> \(M_{C_nH_{2n+2}}=\dfrac{3,6}{\dfrac{0,25}{n}}=14,4n\left(g/mol\right)\)
=> n = 5
=> CTPT: C5H12
CTCT:
(1) \(CH_3-CH_2-CH_2-CH_2-CH_3\) (pentan)
(2) \(CH_3-CH_2-CH\left(CH_3\right)-CH_3\) (2-metylbutan)
(3) \(\left(CH_3\right)_4C\) (2,2-đimetylpropan)
b) X tác dụng với clo thu được 1 sp thế duy nhất
=> X là (CH3)4C
Câu 5:
\(n_{Br_2}=\dfrac{24}{160}=0,15\left(mol\right)\)
=> \(n_{anken}=0,15\left(mol\right)\)
=> \(M_{anken}=\dfrac{8,4}{0,15}=56\left(g/mol\right)\)
=> X là C4H8
CTCT
(1) \(CH_2=CH-CH_2-CH_3\) (But-1-en)
(2) \(CH_3-CH=CH-CH_3\) (But-2-en)
(3) \(\left(CH_3\right)_2C=CH_2\) (2-metylprop-1-en)
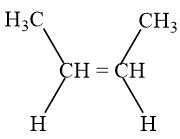
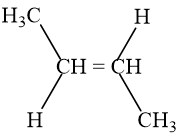
- đphh
(1) 
(2) 
b) X tác dụng với HCl chỉ thu được 1 sp
=> X là \(CH_3-CH=CH-CH_3\)
\(CH_3-CH=CH-CH_3+HCl\rightarrow CH_3-CH_2=CHCl-CH_3\)
Câu 6:
a)
C2H4 + Br2 --> C2H4Br2
C2H2 + 2Br2 --> C2H2Br4
\(C_2H_2+2AgNO_3+2NH_3\rightarrow C_2Ag_2\downarrow+2NH_4NO_3\)
b) TN1: Khí thoát ra khỏi bình là C2H6
=> \(V_{C_2H_6}=4,48\left(l\right)\)
TN2:
\(n_{C_2Ag_2}=\dfrac{36}{240}=0,15\left(mol\right)\)
=> \(n_{C_2H_2}=0,15\left(mol\right)\)
=> \(V_{C_2H_2}=0,15.22,4=3,36\left(l\right)\)
=> \(V_{C_2H_4}=11,2-4,48-3,36=3,36\left(l\right)\)




















