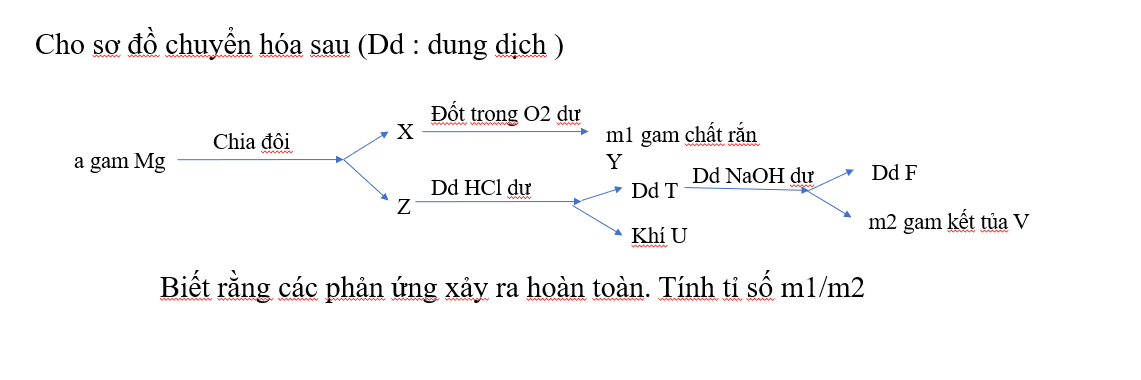
\(2Mg+O_2\xrightarrow{t^o}2MgO\\ Mg+2HCl\to MgCl_2+H_2\\ MgCl_2+2NaOH\to Mg(OH)_2+2NaCl\)
Do đó: \(\begin{cases} X,Z:Mg\\ Y:MgO\\ T:MgCl_2\\ U:H_2\\ F:NaCl\\ V:Mg(OH)_2 \end{cases}\)
Ta có:
\(n_{Mg}=\dfrac{a}{24}\Rightarrow n_{MgO}=\dfrac{a}{48}\\ \Rightarrow m_1=m_{MgO}=\dfrac{a}{48}.40=\dfrac{5a}{6}\\ n_{MgCl_2}=\dfrac{a}{48}\Rightarrow n_{Mg(OH)_2}=\dfrac{a}{48}\\ \Rightarrow m_2=m_{Mg(OH)_2}=\dfrac{a}{48}.58=\dfrac{29a}{24}\\ \Rightarrow \dfrac{m_1}{m_2}=\dfrac{5}{6}:\dfrac{29}{24}=\dfrac{20}{29}\)