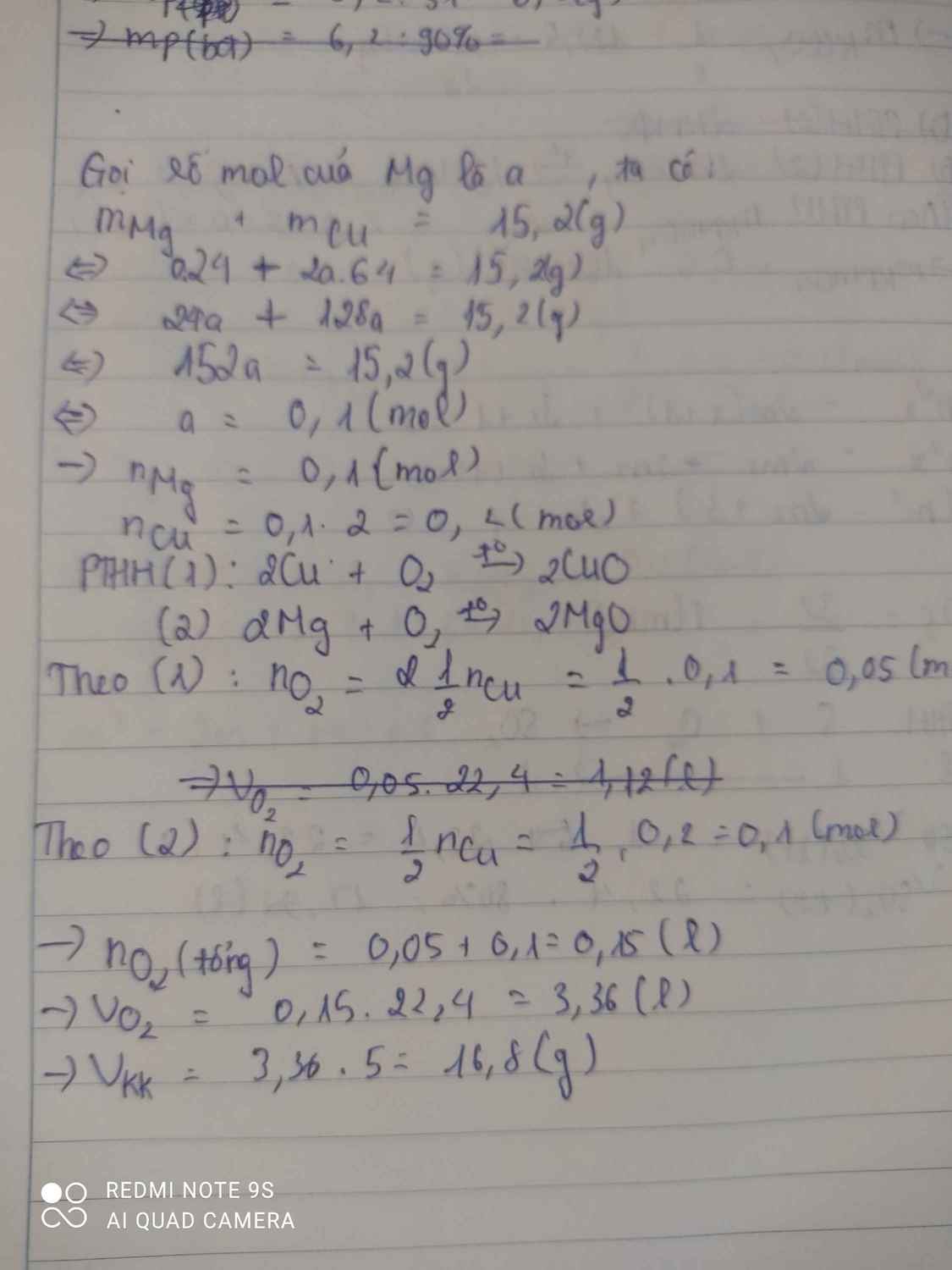PTHH: 2Cu + O2 ---to→ 2CuO
Mol: x 0,5x
PTHH: 2Mg + O2 ---to→ 2MgO
Mol: 0,5x 0,25x (do số mol của Cu gấp đôi Mg)
Ta có: \(64x+24.0,25x=15,2\Leftrightarrow x=\dfrac{38}{175}\left(mol\right)\)
\(\Rightarrow n_{O_2}=0,5.\dfrac{38}{175}+0,25.\dfrac{38}{175}=\dfrac{57}{350}\left(mol\right)\)
\(\Rightarrow V_{O_2}=\dfrac{57}{350}.22,4=3,648\left(l\right)\)
\(\Rightarrow V_{kk}=3,648.5=18,24\left(l\right)\)
PTHH : \(2Cu+O_2\left(t^o\right)->2CuO\) (1)
\(2Mg+O_2\left(t^o\right)->2MgO\) (2)
Gọi x là số mol của Mg lak x (x ∈ Z+)
Theo đề ra : \(n_{Cu}=2n_{Mg}\)
Lại có : \(m_{Cu}+m_{Mg}=15,2\left(g\right)\)
-> \(n_{Cu}.M_{Cu}+n_{Mg}.M_{Mg}=15,2\)
-> \(2.n_{Mg}.M_{Cu}+n_{Mg}.M_{Mg}=15,2\)
-> \(2.n_{Mg}.64+n_{Mg}.24=15,2\)
-> \(n_{Mg}.\left(128+24\right)=15,2\)
-> \(n_{Mg}=0.1\left(mol\right)\)
Vậy \(n_{Cu}=0.2\left(mol\right)\)
Từ (1) => \(\dfrac{1}{2}n_{Cu}=n_{O_2}=0.1\left(mol\right)\)
=> \(V_{O_2\left(1\right)}=n.22,4=2,24\left(l\right)\)
Từ (2) => \(\dfrac{1}{2}n_{Mg}=n_{O_2}=0.05\left(mol\right)\)
=> \(V_{O_2\left(2\right)}=n.22,4=1,12\left(l\right)\)
=> \(V_{O_2\left(PƯ\right)}=V_{O_2\left(1\right)}+V_{O_2\left(2\right)}=1,12+2,24=3,36\left(l\right)\)