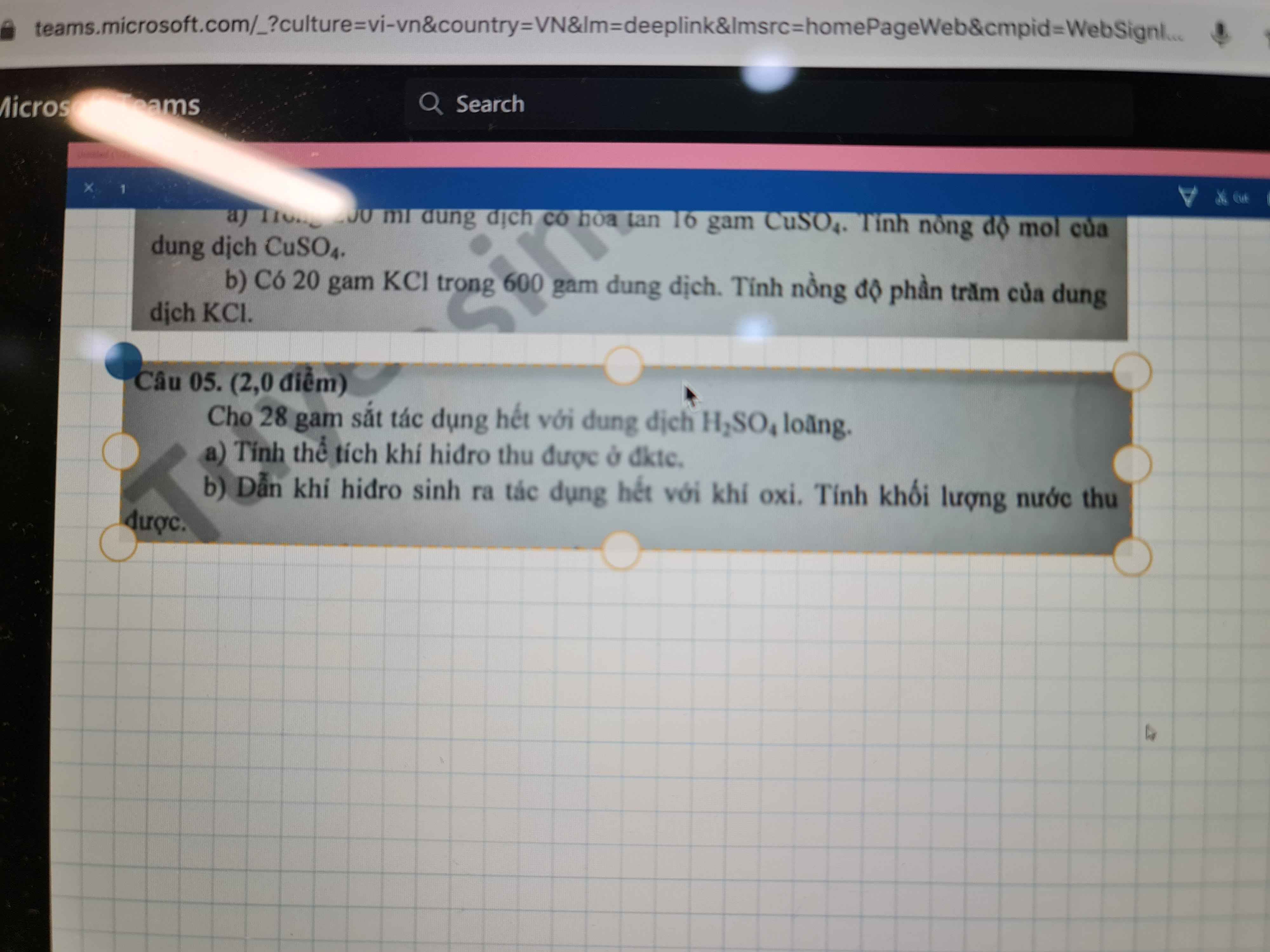
Bai 4 :
\(n_{Fe}=\dfrac{0,84}{56}=0,015\left(mol\right)\)
Pt : \(3Fe+2O_2\underrightarrow{t^o}Fe_3O_4|\)
3 2 1
0,015 0,01 0,005
a) \(n_{O2}=\dfrac{0,015.2}{3}=0,01\left(mol\right)\)
\(V_{O2\left(dktc\right)}=0,01.22,4=0,224\left(l\right)\)
b) \(n_{Fe3O4}=\dfrac{0,01.1}{2}=0,005\left(mol\right)\)
⇒ \(m_{Fe3O4}=0,005.232=1,16\left(g\right)\)
Chuc ban hoc tot
Cau 5 :
\(n_{Fe}=\dfrac{0,28}{56}=0,005\left(mol\right)\)
Pt : \(3H_2+Fe_2O_3\underrightarrow{t^o}2Fe+3H_2O|\)
3 1 2 3
0,0075 0,0025 0,005 0,0075
a) \(n_{Fe2O3}=\dfrac{0,005.1}{2}=0,0025\left(mol\right)\)
⇒ \(m_{Fe2O3}=0,0025.160=0,4\left(g\right)\)
b) \(n_{H2}=\dfrac{0,0025.3}{1}=0,0075\left(mol\right)\)
\(V_{H2\left(dktc\right)}=0,0075.22,4=0,168\left(l\right)\)
c) \(n_{H2O}=\dfrac{0,005.3}{2}=0,0075\left(mol\right)\)
⇒ \(m_{H2O}=0,0075.18=0,135\left(g\right)\)
Chuc ban hoc tot