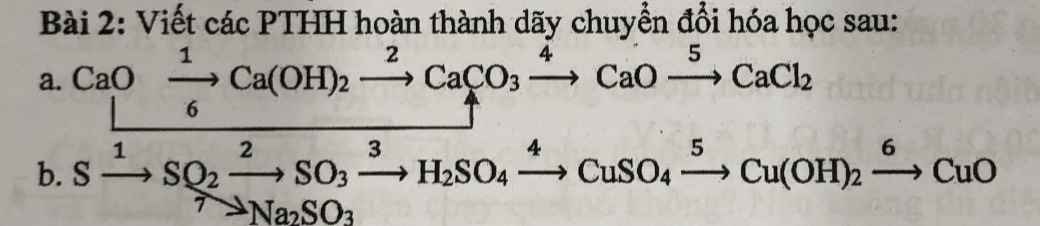
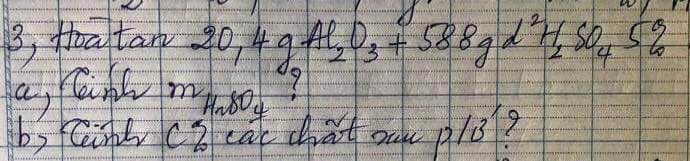Bài 1:
PTHH: \(K_2CO_3+2HCl\rightarrow2KCl+CO_2\uparrow+H_2O\)
a_____2a______2a______a (mol)
\(Na_2CO_3+2HCl\rightarrow2NaCl+CO_2\uparrow+H_2O\)
b_____2b_______2b______b (mol)
a) Ta lập HPT: \(\left\{{}\begin{matrix}138a+106b=4,72\\a+b=\dfrac{0,896}{22,4}=0,04\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,015\\b=0,025\end{matrix}\right.\)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{K_2CO_3}=\dfrac{138\cdot0,015}{4,72}\cdot100\%\approx43,86\%\\\%m_{Na_2CO_3}=56,14\%\end{matrix}\right.\)
b+c) Theo các PTHH: \(\left\{{}\begin{matrix}n_{NaCl}=0,05\left(mol\right)\\n_{KCl}=0,03\left(mol\right)\\n_{HCl}=0,08\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{muối}=0,05\cdot58,5+0,03\cdot74,5=5,16\left(g\right)\\C_{M_{HCl}}=\dfrac{0,08}{0,1}=0,8\left(M\right)\end{matrix}\right.\)
Bài 2:
Ta có: \(\left\{{}\begin{matrix}n_{Mg}=\dfrac{0,6}{24}=0,025\left(mol\right)\\n_{H_2\left(axit\:\right)}=\dfrac{1,568}{22,4}=0,07\left(mol\right)\end{matrix}\right.\)
PTHH: \(Al+NaOH+H_2O\rightarrow NaAlO_2+\dfrac{3}{2}H_2\)
\(Mg+H_2SO_4\rightarrow MgSO_4+H_2\uparrow\)
0,025____________________0,025 (mol)
\(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\uparrow\)
0,03_______________________0,045 (mol)
\(\Rightarrow\left\{{}\begin{matrix}\%m_{Mg}=\dfrac{0,6}{0,6+0,03\cdot27}\cdot100\%\approx42,55\%\\\%m_{Al}=57,45\%\end{matrix}\right.\)









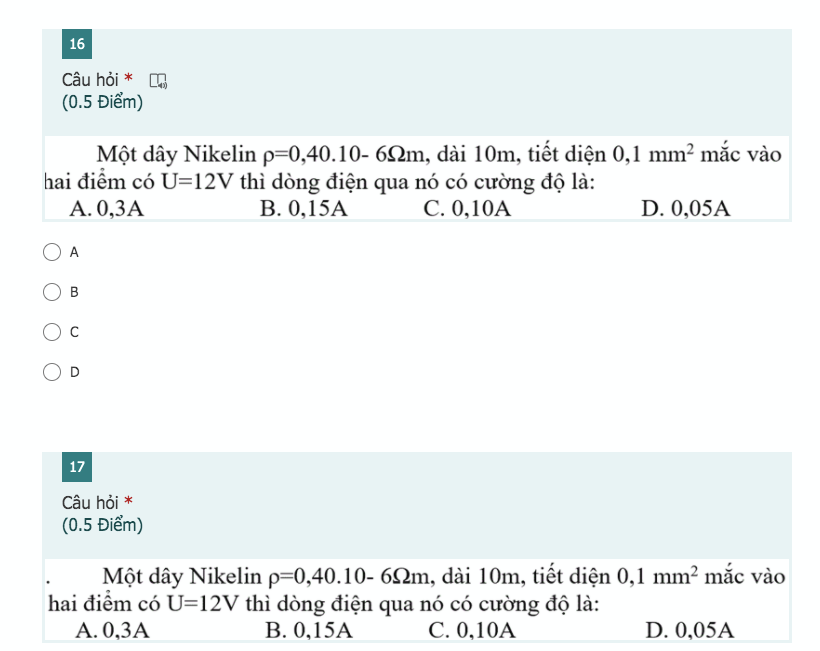 mọi người giúp em với em cần gấp <3
mọi người giúp em với em cần gấp <3