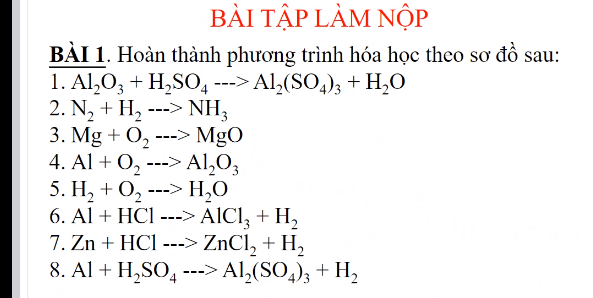
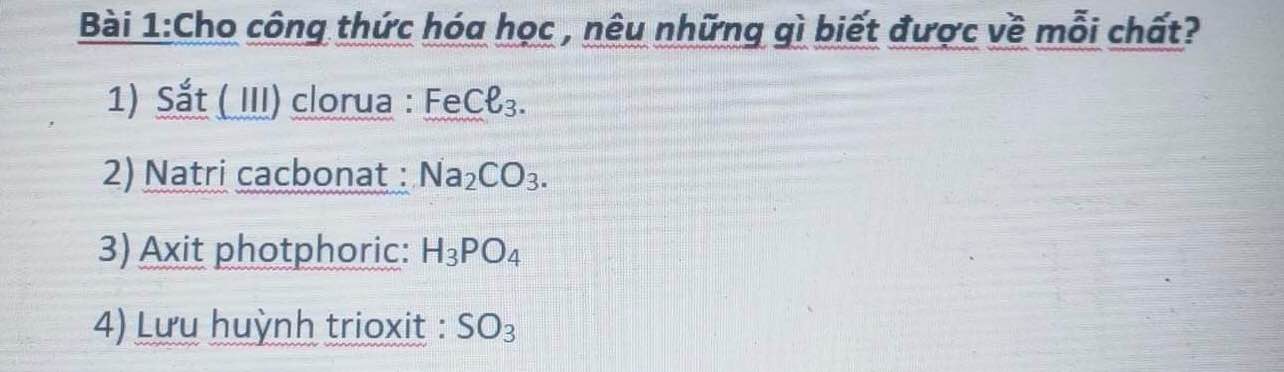
PTHH: 2KClO3 --to--> 2KCl + 3O2
\(n_{KCl}=\dfrac{45}{74,5}=\dfrac{90}{149}\left(mol\right)\)
PTHH: 2KClO3 --to--> 2KCl + 3O2
___________________\(\dfrac{90}{149}\)--> \(\dfrac{135}{149}\)___(mol)
=> mO2 = \(\dfrac{135}{149}.32\approx29\left(g\right)\)