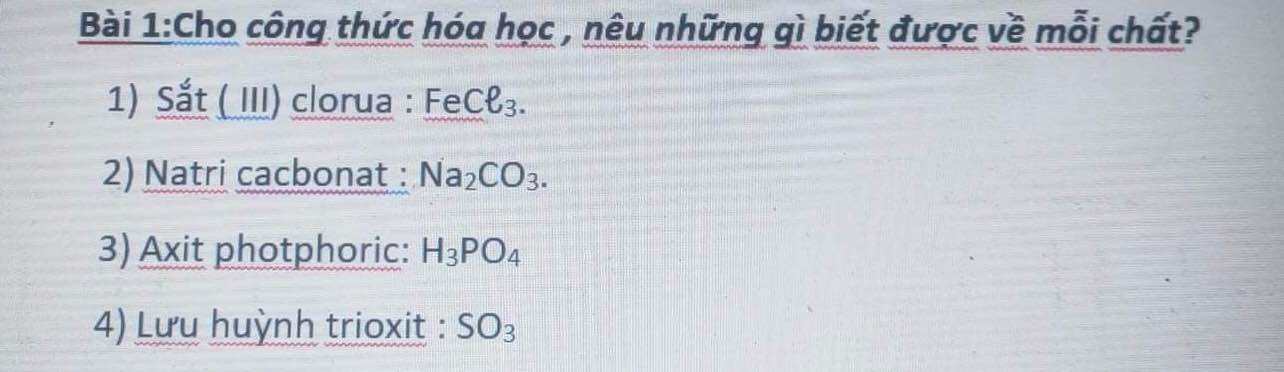
bài 5:
theo đề bài ta có:
\(p+n+e=155\)
mà \(p=e\)
\(\Rightarrow\left\{{}\begin{matrix}2p+n=155\\2p-n=33\end{matrix}\right.\)
\(\Leftrightarrow\left\{{}\begin{matrix}2n=122\\2p-n=33\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}n=61\\2p-61=33\end{matrix}\right.\Leftrightarrow\left\{{}\begin{matrix}n=61\\p=47\end{matrix}\right.\)
vậy \(p=e=47;n=61\)
\(NTK=47+61=108\left(đvC\right)\)
\(\Rightarrow\) nguyên tố cần tìm là bạc \(\left(Ag\right)\)
Bài 1:
a) HNO3
b) C3H8
c) CaCO3
d) C2H4O2
e) C2H6O
f) CO2
Bài 2:
a) CTHH: C2H6 PTK: 12.2 + 1.6 = 30 đvC
b) CTHH: Al2O3 PTK: 27.2 + 16.3 = 102 đvC
c) CTHH: K NTK: 39 đvC
d) CTHH: Na(OH) PTK: 23.1 + (16.1 + 1.1) = 40 đvC
e) CTHH: Cl NTK: 35.5 đvC
f) CTHH: O3 PTK: 16.3 = 48 đvC
g) CTHH: H2(SO4) PTK: 1.2 + (32.1 + 16.4) = 98 đvC
h) CTHH: Si NTK: 28 đvC
i) CTHH: C12H22O11 PTK: 12.12 + 1.22 + 16.11 = 342 đvC
j) CTHH: Ni NTK: 14 đvC
k) CTHH: C PTK: 12