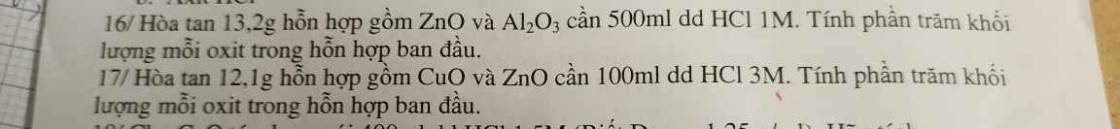\(16/n_{HCl}=0,5.1=0,5mol\\ n_{ZnO}=a;n_{Al_2O_3}=b\\ ZnO+2HCl\rightarrow ZnCl_2+H_2O\\ Al_2O_3+6HCl\rightarrow2AlCl_3\rightarrow3H_2O\\ \Rightarrow\left\{{}\begin{matrix}81a+102b=13,2\\2a+6b=0,5\end{matrix}\right.\\ \Rightarrow a=0,1;b=0,05\\ \%m_{ZnO}=\dfrac{0,1.81}{13,2}\cdot100=61,36\%\\ \%m_{Al_2O_3}=100-61,36=38,64\%\)
Đúng 3
Bình luận (0)
\(17/n_{HCl}=0,1.3=0,3mol\\ n_{CuO}=a;n_{ZnO}=b\\ CuO+2HCl\rightarrow CuCl_2+H_2O\\ ZnO+2HCl\rightarrow ZnCl_2+H_2O\\ \Rightarrow\left\{{}\begin{matrix}80a+81b=12,1\\2a+2b=0,3\end{matrix}\right.\\ \Rightarrow a=0,05;b=0,1\\ \%m_{CuO}=\dfrac{0,05.80}{12,1}\cdot100=33,06\%\\ \%m_{ZnO}=100-33,06=66,94\%\)
Đúng 2
Bình luận (0)