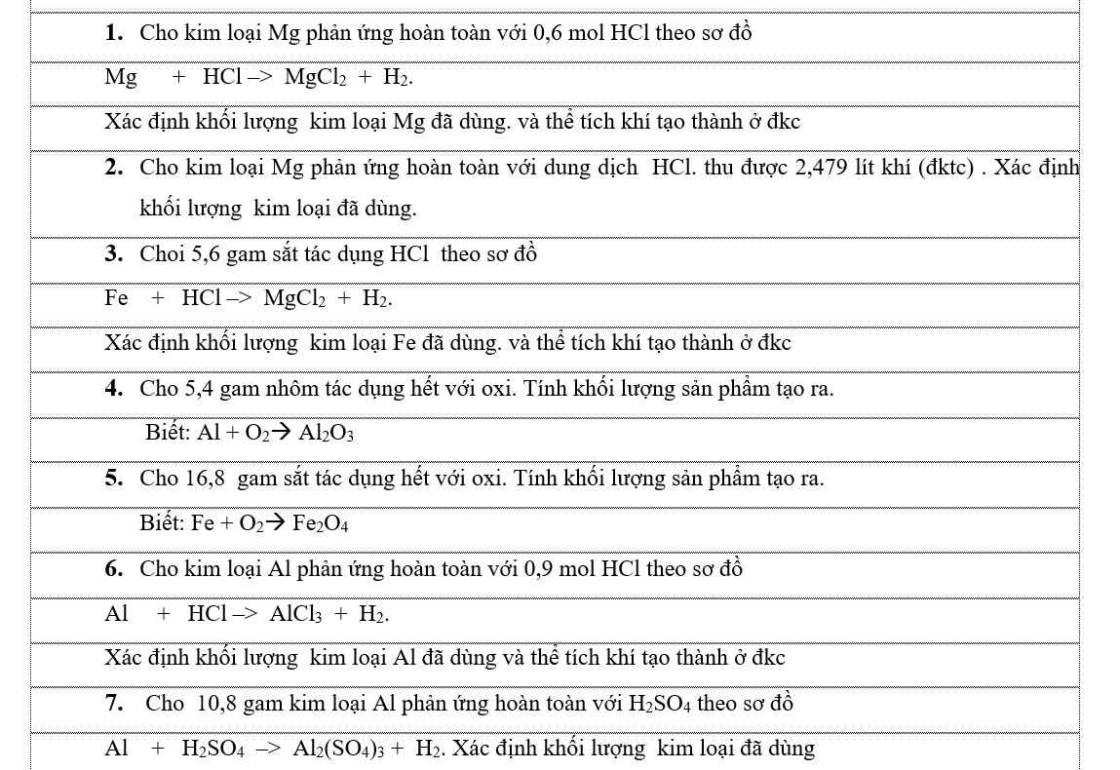
\(2.\\ n_{H_2}=\dfrac{2,479}{22,4}=\dfrac{2479}{22400}mol\\ Mg+2HCl\rightarrow MgCl_2+H_2\\ n_{Mg}=n_{H_2}=\dfrac{2479}{22400}mol\\ m_{Mg}=\dfrac{2479}{22400}\cdot24=2,56g\)
\(3.\\ n_{Fe}=\dfrac{5,6}{56}=0,1mol\\ Fe+2HCl\rightarrow FeCl_2+H_2\\ m_{Fe}=5,6g\\ n_{H_2}=n_{Fe}=0,1mol\\ V_{H_2}=0,1.22,4=2,24l\)
\(4. \\ n_{Al}=\dfrac{5,4}{27}=0,2mol\\4 Al+3O_2\xrightarrow[]{t^0}2Al_2O_3\\ n_{Al_2O_3}=0,2:2=0,1mol\\ m_{Al_2O_3}=0,1.102=10,2g\)
\(5.\\ n_{Fe}=\dfrac{16,8}{56}=0,3mol\\4 Fe+3O_2\xrightarrow[]{t^0}2Fe_2O_3\\ n_{Fe_2O_3}=0,3:4.2=0,15mol\\ m_{Fe_2O_3}=0,15.160=24g\)
\(1.Mg+2HCl\xrightarrow[]{}MgCl_2+H_2O\\ n_{Mg}=\dfrac{1}{2}0,6=0,3mol\\ m_{Mg}=0,3.24=7,2g\)
\(7.n_{Al}=\dfrac{10,8}{27}=0,4mol\\ 2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\\ m_{Al}=10,8g\)