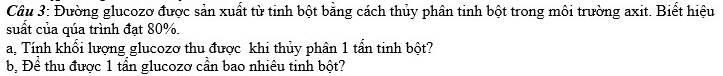`C5:`
`n_[CO_2]=[2,24]/[22,4]=0,1(mol)`
`C_6 H_12 O_6 -\text{lên men}->2C_2 H_5 OH+2CO_2`
`0,05` `0,1` `0,1` `(mol)`
`a)m_[C_2 H_5 OH]=0,1.46=4,6(g)`
`b)m_[C_6 H_12 O_6]=[0,05.100.180]/80=11,25(g)`
`C3:`
`C_6 H_10 O_5 +H_2 O-H^[+],t^[o]->C_6 H_12 O_6`
`a)m_[C_6 H_12 O_6]=[1000.80]/[162.100]=4,94(g)`
`b)m_[C_6 H_10 O_5]=[1000.100]/[180.80]=6,94(g)`