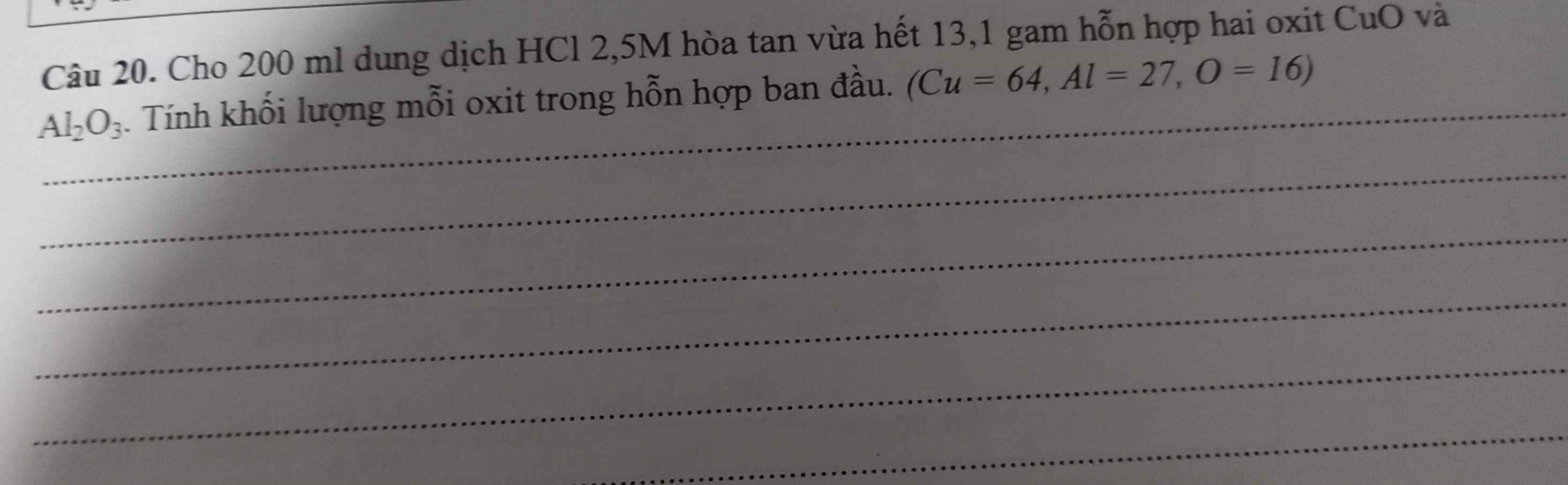`CuO + 2HCl -> CuCl_2 + H_2 O`
`Al_2 O_3 + 6HCl -> 2AlCl_3 + 3H_2 O`
`n_[HCl]=2,5.0,2=0,5(mol)`
Gọi `n_[CuO]=x;n_[Al_2 O_3]=y`
`=>` $\begin{cases}80x+102y=13,1\\2x+6y=0,5 \end{cases}$
`<=>` $\begin{cases} x=0,1\\y=0,05 \end{cases}$
`m_[CuO]=0,1.80=8(g)`
`=>m_[Al_2 O_3]=13,1-8=5,1(g)`
Gọi \(\left\{{}\begin{matrix}n_{CuO}=x\left(mol\right)\\n_{Al_2O_3}=y\left(mol\right)\end{matrix}\right.\Rightarrow80x+102y=13,1\left(1\right)\)
PTHH: \(CuO+2HCl\rightarrow CuCl_2+H_2O\)
x-------->2x
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
y-------->6y
`=> 2x + 6y = 0,2.2,5 = 0,5 (2)`
`(1), (2) => x = 0,1; y = 0,05`
`=> m_{CuO} = 0,1.80 = 8(g); m_{Al_2O_3} = 0,05.102 = 5,1 (g)`