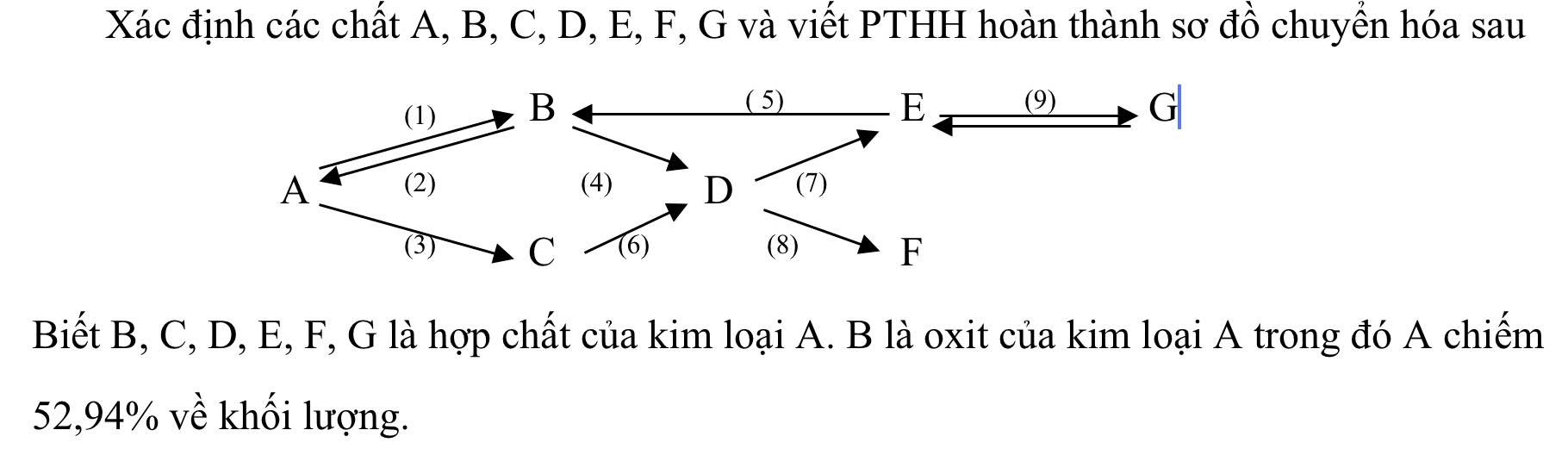
CTHH của B là A2On
Ta có: \(\dfrac{\%A}{\%O}=\dfrac{2.M_A}{16n}=\dfrac{52,94\%}{47,06\%}\Rightarrow M_A=9n\left(g/mol\right)\)
Xét n = 3 thỏa mãn => MA = 27 (g/mol)
=> A là Al
B: Al2O3
C: Al2(SO4)3
D: AlCl3
E: Al(OH)3
F: Al(NO3)3
G: NaAlO2
(1) \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
(2) \(2Al_2O_3\underrightarrow{đpnc,criolit}4Al+3O_2\)
(3) \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
(4) \(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
(5) \(2Al\left(OH\right)_3\underrightarrow{t^o}Al_2O_3+3H_2O\)
(6) \(Al_2\left(SO_4\right)_3+3BaCl_2\rightarrow2AlCl_3+3BaSO_4\downarrow\)
(7) \(AlCl_3+3NaOH\rightarrow Al\left(OH\right)_3\downarrow+3NaCl\)
(8) \(AlCl_3+3AgNO_3\rightarrow Al\left(NO_3\right)_3+3AgCl\downarrow\)
(9) \(Al\left(OH\right)_3+NaOH\rightarrow NaAlO_2+2H_2O\)
(10) \(NaAlO_2+HCl+H_2O\rightarrow NaCl+Al\left(OH\right)_3\)