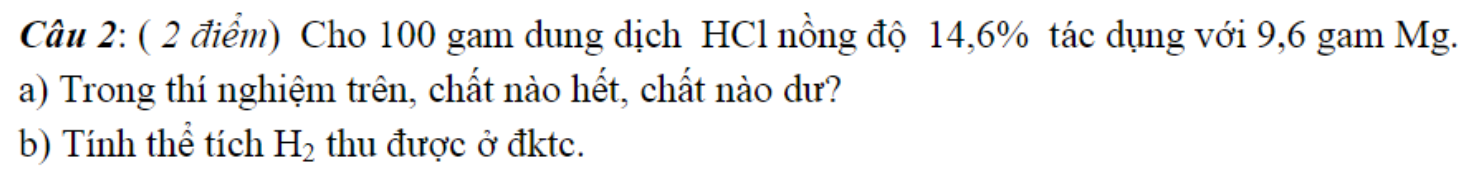
\(n_{Mg}=\dfrac{9,6}{24}=0,4\left(mol\right)\)
\(n_{HCl}=\dfrac{100\times14,6\%}{36,5}=0,4\left(mol\right)\)
\(Mg+2HCl\rightarrow MgCl_2+H_2\)
`0,4` > `0,4` ( mol )
`0,4` `0,2` ( mol )
Chất hết là `HCl`, chất dư là `Mg`
`V_{H_2}=0,2.22,4=4,48(l)`
\(n_{HCl}=\dfrac{\dfrac{100.14,6}{100}}{36,5}=0,4\left(mol\right)\\
n_{Mg}=\dfrac{9,6}{24}=0,4\left(mol\right)\\
pthh:Mg+2HCl\rightarrow MgCl_2+H_2\uparrow\)
\(LTL:\dfrac{0,4}{1}>\dfrac{0,4}{2}\)
=> Mg dư , HCl hết
\(n_{H_2}=\dfrac{1}{2}n_{HCl}=0,2\left(mol\right)\\
V_{H_2}=0,2.22,4=8,96\left(l\right)\)