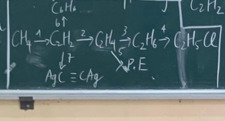(1) \(2CH_4\underrightarrow{1500^oC}C_2H_2+3H_2\)
(2) \(C_2H_2+H_2\underrightarrow{t^o,Pd/PbCO_3}C_2H_4\)
(3) \(C_2H_4+H_2\underrightarrow{t^o,Ni}C_2H_6\)
(4) \(C_2H_6+Cl_2\underrightarrow{askt}C_2H_5Cl+HCl\)
(5) \(nC_2H_4\underrightarrow{t^o,p,xt}\left(-CH_2-CH_2-\right)_n\)
(6) \(3C_2H_2\underrightarrow{trime.hóa}C_6H_6\)
(7) \(C_2H_2+2AgNO_3+2NH_3\rightarrow C_2Ag_2+2NH_4NO_3\)
\(\left(1\right)2CH_4\rightarrow\left(1500^oC,làm.lạnh.nhanh\right)C_2H_2+3H_2\\ \left(2\right)C_2H_2+H_2\rightarrow\left(t^o,\dfrac{Pd}{PbCO_3}\right)C_2H_4\\ \left(3\right)C_2H_4+H_2\rightarrow\left(Ni,t^o\right)C_2H_6\\ \left(5\right)C_2H_6+Cl_2\rightarrow\left(as\right)C_2H_5Cl+HCl\\ \left(5\right)nCH_2=CH_2\rightarrow\left(t^o,P,xt\right)\left(-CH_2-CH_2-\right)_n\\ \left(6\right)CH\equiv CH+2AgNO_3+2NH_3\rightarrow AgC\equiv CAg+2NH_4NO_3\)