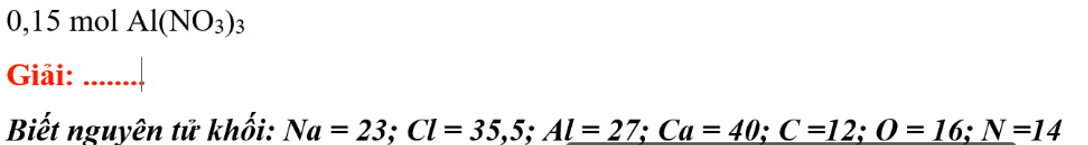\(M=27+\left(14+16.3\right).3=213\left(\dfrac{g}{mol}\right)\)
\(m=n.M=0,15.213=31,95\left(g\right)\)
\(m_{Al\left(NO_3\right)_3}=0,15.213=31,95\left(g\right)\)
\(V_{Al\left(NO_3\right)_3}=0,15.22,4=3,36\left(l\right)\)
mk k bt đề bài hỏi j nên mk làm 2 cách nha
\(m_{Al\left(NO_3\right)_3}=0,15.\left[27+\left(12+16.3\right).3\right]=0,15.207=31,05\left(g\right)\)