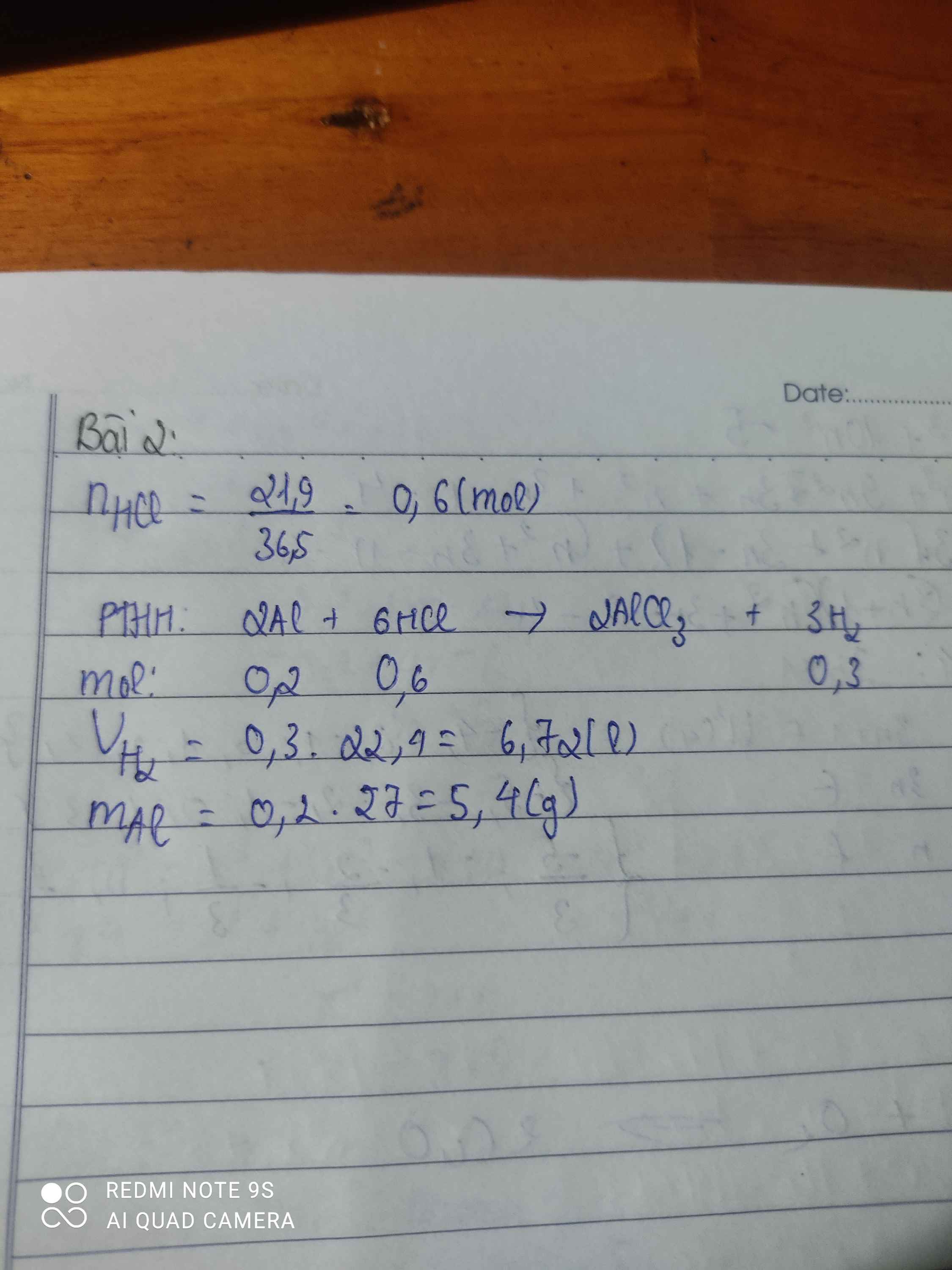\(n_{HCl}=\dfrac{21,9}{36,5}=0,6\left(mol\right)\\ a,PTHH:2Al+6HCl\rightarrow2AlCl_3+3H_2\\ n_{H_2}=\dfrac{3}{6}.0,6=0,3\left(mol\right)\\ \Rightarrow V_{H_2\left(đktc\right)}=0,3.22,4=6,72\left(l\right)\\ n_{Al}=\dfrac{2}{6}.0,6=0,2\left(mol\right)\\ \Rightarrow a=m_{Al}=0,2.27=5,4\left(g\right)\)
a)\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
b)\(n_{HCl}=\dfrac{21,9}{36,5}=0,6\)
\(2Al+6HCl\rightarrow2AlCl_3+3H_2\)
(mol) 1,8 0,6 -> 1,2
\(V_{H_2}=1,2\times22,4=26,88\)
c) \(m_{Al}=27\times1,8=48,6\left(g\right)\)