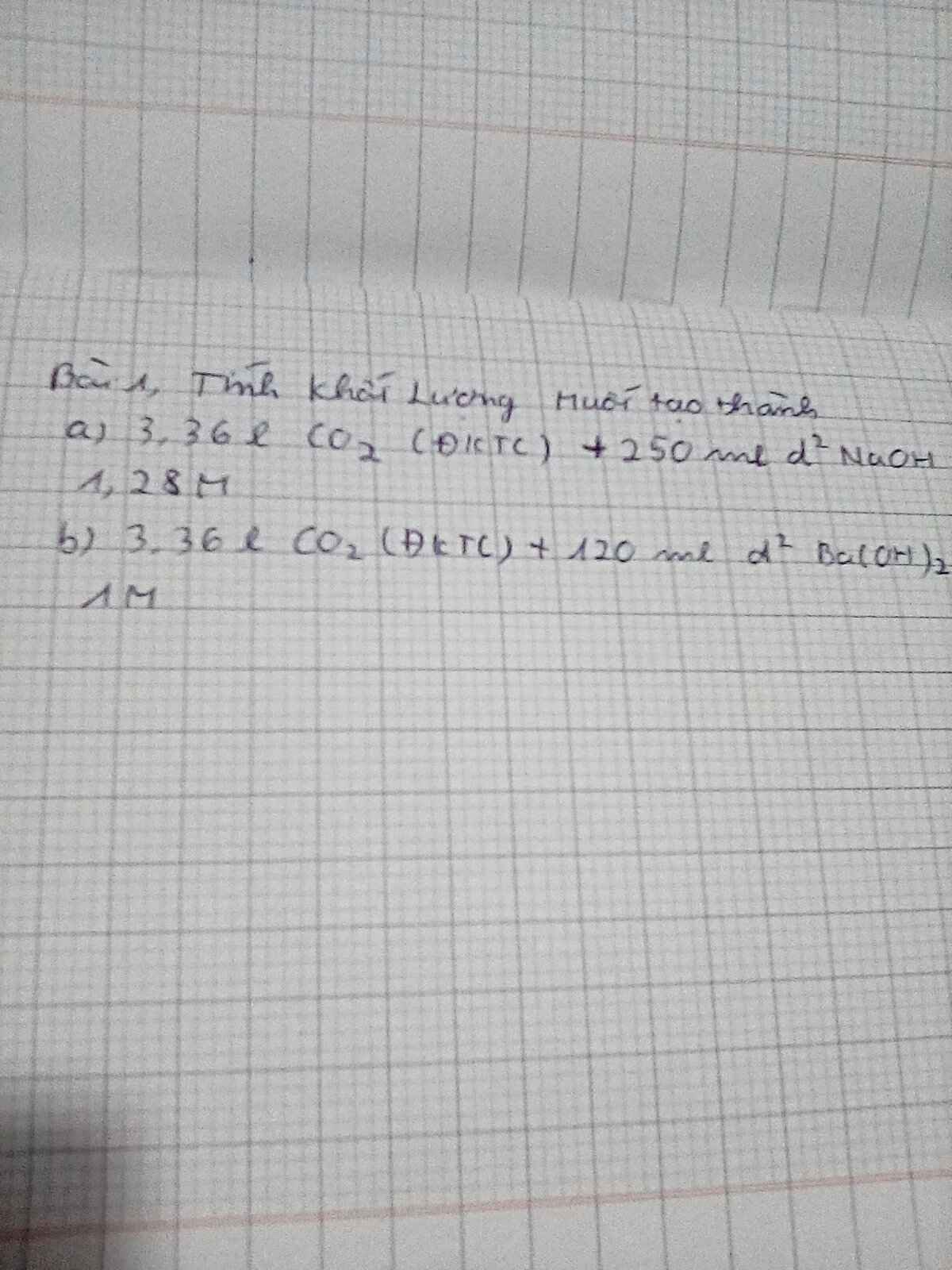a) Ta có: \(\left\{{}\begin{matrix}n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\n_{NaOH}=0,25\cdot1,28=0,32\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow\) Tạo muối trung hòa, bazơ dư \(\Rightarrow\) Tính theo CO2
Bảo toàn Cacbon: \(n_{Na_2CO_3}=n_{CO_2}=0,15\left(mol\right)\) \(\Rightarrow m_{Na_2CO_3}=0,15\cdot106=15,9\left(g\right)\)
b) Ta có: \(\left\{{}\begin{matrix}n_{CO_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\\n_{Ba\left(OH\right)_2}=0,12\left(mol\right)\end{matrix}\right.\) \(\Rightarrow\) Tạo 2 muối
PTHH: \(CO_2+Ba\left(OH\right)_2\rightarrow BaCO_3+H_2O\)
a_________a_________a (mol)
\(2CO_2+Ba\left(OH\right)_2\rightarrow Ba\left(HCO_3\right)_2\)
2b_________b_____________b (mol)
Ta lập HPT: \(\left\{{}\begin{matrix}a+2b=0,15\\a+b=0,12\end{matrix}\right.\) \(\Leftrightarrow\left\{{}\begin{matrix}a=0,09\\b=0,03\end{matrix}\right.\) \(\Rightarrow\left\{{}\begin{matrix}m_{BaCO_3}=0,09\cdot197=17,73\left(g\right)\\m_{Ba\left(HCO_3\right)_2}=0,03\cdot259=7,77\left(g\right)\end{matrix}\right.\)