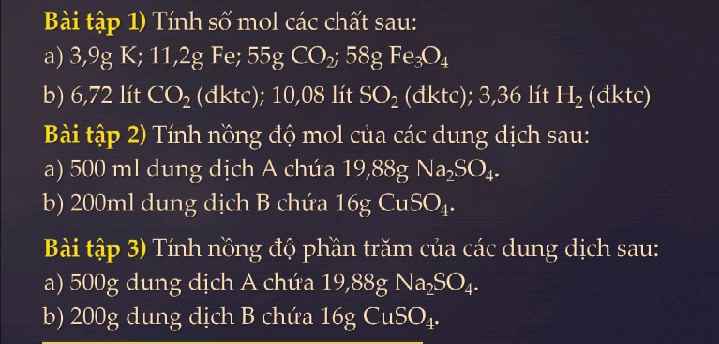
Bài 1:
\(a.n_K=\dfrac{3,9}{39}=0,1\left(mol\right)\\ n_{Fe}=\dfrac{11,2}{56}=0,2\left(mol\right)\\ n_{CO_2}=\dfrac{55}{44}=1,25\left(mol\right)\\ n_{Fe_3O_4}=\dfrac{58}{232}=0,25\left(mol\right)\\ b.n_{CO_2}=\dfrac{6,72}{22,4}=0,3\left(mol\right)\\ n_{SO_2}=\dfrac{10,08}{22,4}=0,45\left(mol\right)\\ n_{H_2}=\dfrac{3,36}{22,4}=0,15\left(mol\right)\)
Bài 2:
\(a.n_{Na_2SO_4}=\dfrac{19,88}{142}=0,14\left(mol\right)\\ C_{MddNa_2SO_4}=\dfrac{0,14}{0,5}=0,28\left(M\right)\\ b.n_{CuSO_4}=\dfrac{16}{160}=0,1\left(mol\right)\\ C_{MddCuSO_4}=\dfrac{0,1}{0,2}=0,5\left(M\right)\)
Bài 3:
\(a.C\%_{ddA}=C\%_{ddNa_2SO_4}=\dfrac{19,88}{500}.100=3,976\%\\ b.C\%_{ddCuSO_4}=\dfrac{16}{200}.100=8\%\)