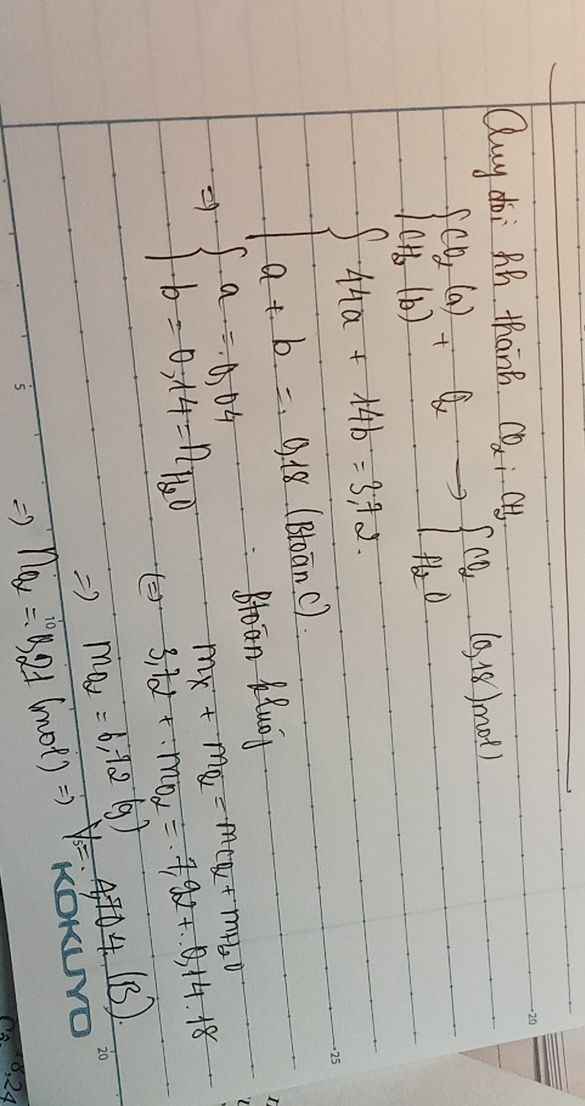Qui E gồm CH2 a mol, CO2 b mol
mE = 14a + 44b = 3,72
Mol CO2 = a + b = 0,18
=> a = 0,14 và b = 0,04
Mol O2 = 1,5a = 0,21 => V = 4,704 lít
Gọi: \(\left\{{}\begin{matrix}n_E=a\left(mol\right)\\n_{O_2}=b\left(mol\right)\\n_{H_2O}=c\left(mol\right)\end{matrix}\right.\)
Có: \(n_{CO_2}=0,18\left(mol\right)\)
BTKL, có: 3,72 + 32b = 7,92 + 18c
BTNT O, có: 2a + 2b = 0,18.2 + c
E có k = 2 \(\Rightarrow\dfrac{0,18-c}{k-1}=a\Leftrightarrow0,18-c=a\)
\(\Rightarrow\left\{{}\begin{matrix}a=0,04\left(mol\right)\\b=0,21\left(mol\right)\\c=0,14\left(mol\right)\end{matrix}\right.\)
\(\Rightarrow V_{O_2}=0,21.22,4=4,704\left(l\right)\)
Bạn tham khảo nhé!
1c khác.
\(E\left\{{}\begin{matrix}C_2H_3O\text{:}xmol\\CH_2\text{:}ymol\end{matrix}\right.\)\(\)\(\Rightarrow\) \(\left\{{}\begin{matrix}43x+14y=3,72\left(g\right)\\2x+y=\dfrac{7,92}{44}=0,18\left(mol\right)\end{matrix}\right.\)\(\Rightarrow\left\{{}\begin{matrix}x=0,08\\y=0,02\end{matrix}\right.\)
BTE \(4n_{O_2}=9x+6y\Rightarrow n_{O_2}=0,21\left(mol\right)\) \(\Rightarrow V=4,704\left(l\right)\)