Ta có: \({{\rm{n}}_{{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}} = \frac{{10}}{{180}} = \frac{1}{{18}}{\rm{ (mol)}}\)
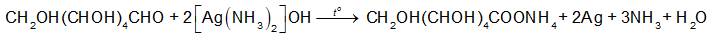
Theo phương trình hóa học: \({{\rm{n}}_{{\rm{Ag}}}}{\rm{ = 2}}{{\rm{n}}_{{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}} = 2 \times \frac{1}{{18}}{\rm{ = }}\frac{1}{9}{\rm{ (mol)}}\)
\( \Rightarrow {{\rm{m}}_{{\rm{Ag}}}}{\rm{ = }}\frac{1}{9} \times {\rm{108}} = 12{\rm{ (g)}}\)
Đúng 0
Bình luận (0)


