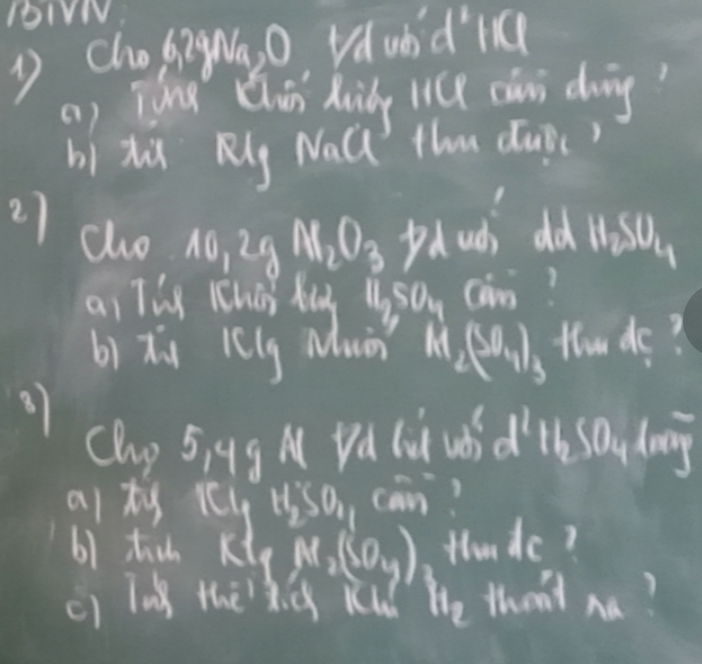1)
\(n_{Na_2O}=\dfrac{6,2}{62}=0,1\left(mol\right)\)
PTHH: \(Na_2O+2HCl\rightarrow2NaCl+H_2O\)
0,1----->0,2----->0,2
a) \(m_{HCl}=0,2.36,5=7,3\left(g\right)\)
b) \(m_{NaCl}=0,2.58,5=11,7\left(g\right)\)
2)
\(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\)
PTHH: \(Al_2O_3+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2O\)
0,1------>0,3---------->0,1
a) \(m_{H_2SO_4}=0,3.98=29,4\left(g\right)\)
b) \(m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2\left(g\right)\)
3)
\(n_{Al}=\dfrac{5,4}{27}=0,2\left(mol\right)\)
PTHH: \(2Al+3H_2SO_4\rightarrow Al_2\left(SO_4\right)_3+3H_2\)
0,2----->0,3---------->0,1------>0,3
a) \(m_{H_2SO_4}=0,3.98=29,4\left(g\right)\)
b) \(m_{Al_2\left(SO_4\right)_3}=0,1.342=34,2\left(g\right)\)
c) \(V_{H_2}=0,3.22,4=6,72\left(l\right)\)