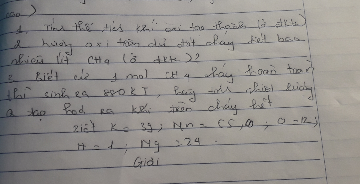\(n_P=\frac{6,2}{31}=0,2\left(mol\right)\)
\(n_{O2}=\frac{7,84}{22,4}=0,35\left(mol\right)\)
a, \(PTHH:4P+5O_2\underrightarrow{^{to}}2P_2O_5\)
________0,2___0,35 mol
Ta thấy :
\(\frac{0,2}{4}< \frac{0,35}{5}\)
\(\Rightarrow n_{O2\left(Dư\right)}=0,35-0,05.5=0,1\left(mol\right)\)
\(\Rightarrow m_{O2\left(Dư\right)}=0,1.32=3,2\left(g\right)\)
b, Theo PT :
\(n_{P2O5}=\frac{1}{2}n_P=0,1\left(mol\right)\)
\(\Rightarrow m_{P2O5}=0,1.142=14,2\left(g\right)\)