\(2KMnO_4\rightarrow K_2MnO_4+MnO_2+O_2\)
a. \(n_{O2}=\frac{24}{32}=0,75\left(mol\right)\)
\(\rightarrow n_{KMnO4}=0,75.2=1,5\)
\(\rightarrow m_{KMnO4}=1,5.\left(39+55+16.4\right)=237\left(g\right)\)
b. \(n_{O2}=\frac{33,6}{22,4}=1,5\)
\(\rightarrow n_{KMnO4}=1,5.2=3\)
\(\rightarrow m_{KMnO4}=3.\left(39+55+16.4\right)=474\left(g\right)\)







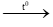 2KCl+3O2
2KCl+3O2


