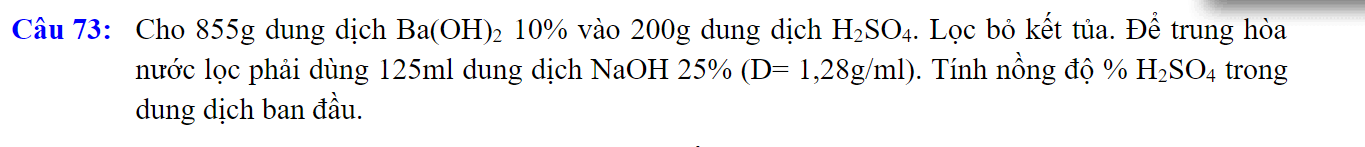\(n_{Ba\left(OH\right)_2}=\dfrac{855.10\%}{171}=0,5\left(mol\right)\)
\(m_{dd.NaOH}=1,28.125=160\left(g\right)\)
=> \(n_{NaOH}=\dfrac{160.25\%}{40}=1\left(mol\right)\)
Gọi số mol H2SO4 là a (mol)
PTHH: Ba(OH)2 + H2SO4 --> BaSO4 + 2H2O
0,5------>0,5
2NaOH + H2SO4 --> Na2SO4 + 2H2O
1----->0,5
=> nH2SO4 = 0,5 + 0,5 = 1 (mol)
=> \(C\%_{dd.H_2SO_4}=\dfrac{1.98}{200}.100\%=49\%\)