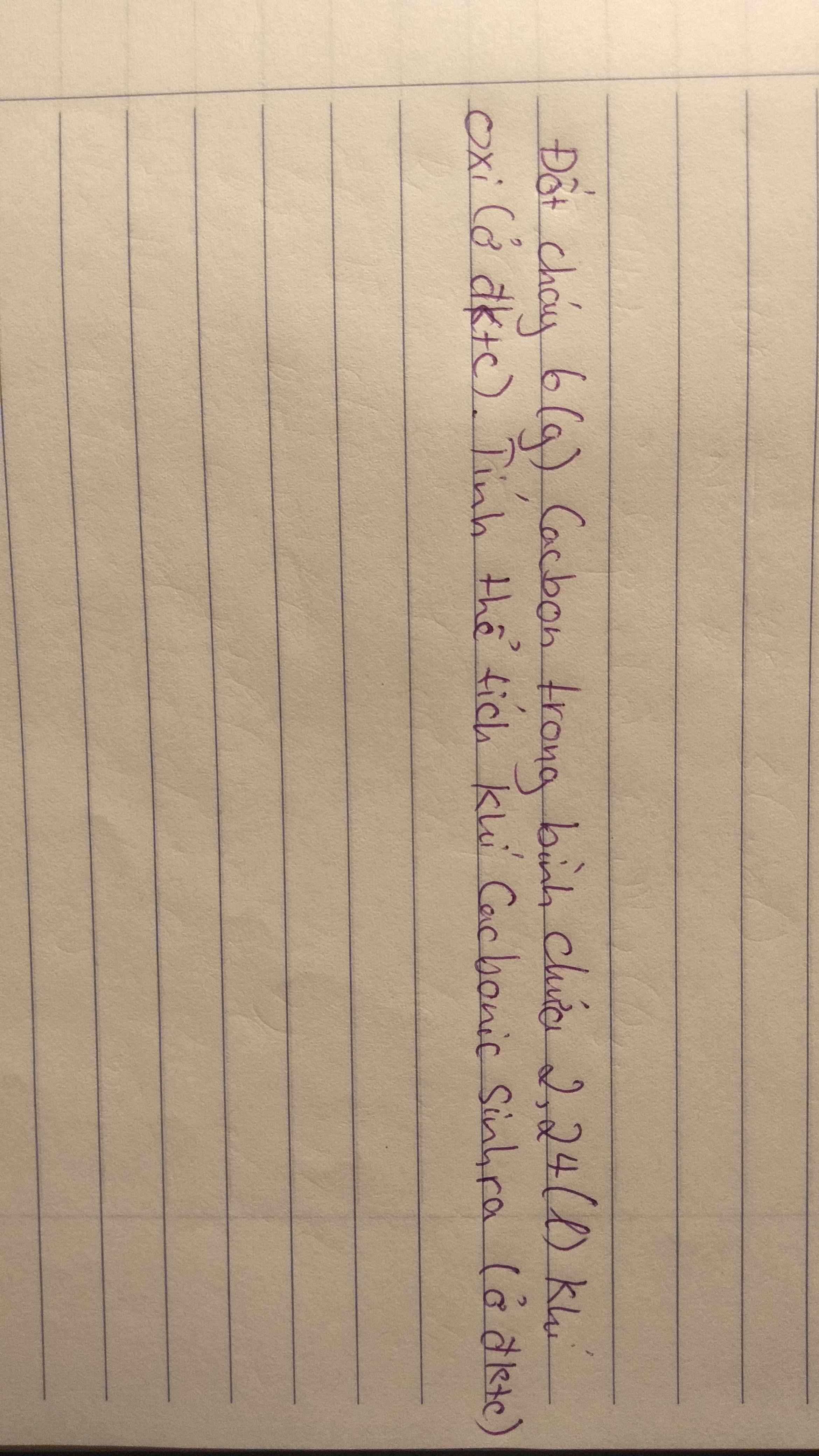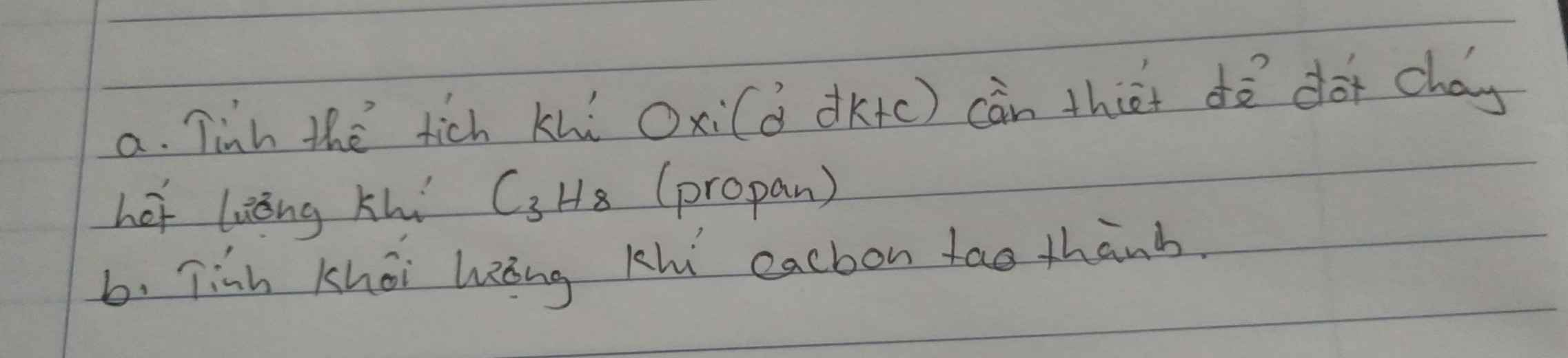Câu 11:
\(\text {Bảo toàn KL: }m_{Fe}+m_{HCl}=m_{FeCl_2}+m_{H_2}\\ \Rightarrow m_{\text{phản ứng}}=m_{Fe_Cl_2}+m_{H_2}=11,61(g)\)
Câu 12:
\(\text{Bảo toàn KL: }m_{Mg}+m_{O_2}=m_{MgO}\\ \Rightarrow m_{O_2}=m_{MgO}-m_{Mg}=15-9=6(g)\)
Câu 13:
\(\text {Bảo toàn KL: }m_{hh}+m_{O_2}=m_{oxit}\\ \Rightarrow m_{O_2}=18-13,2=4,8(g)\)