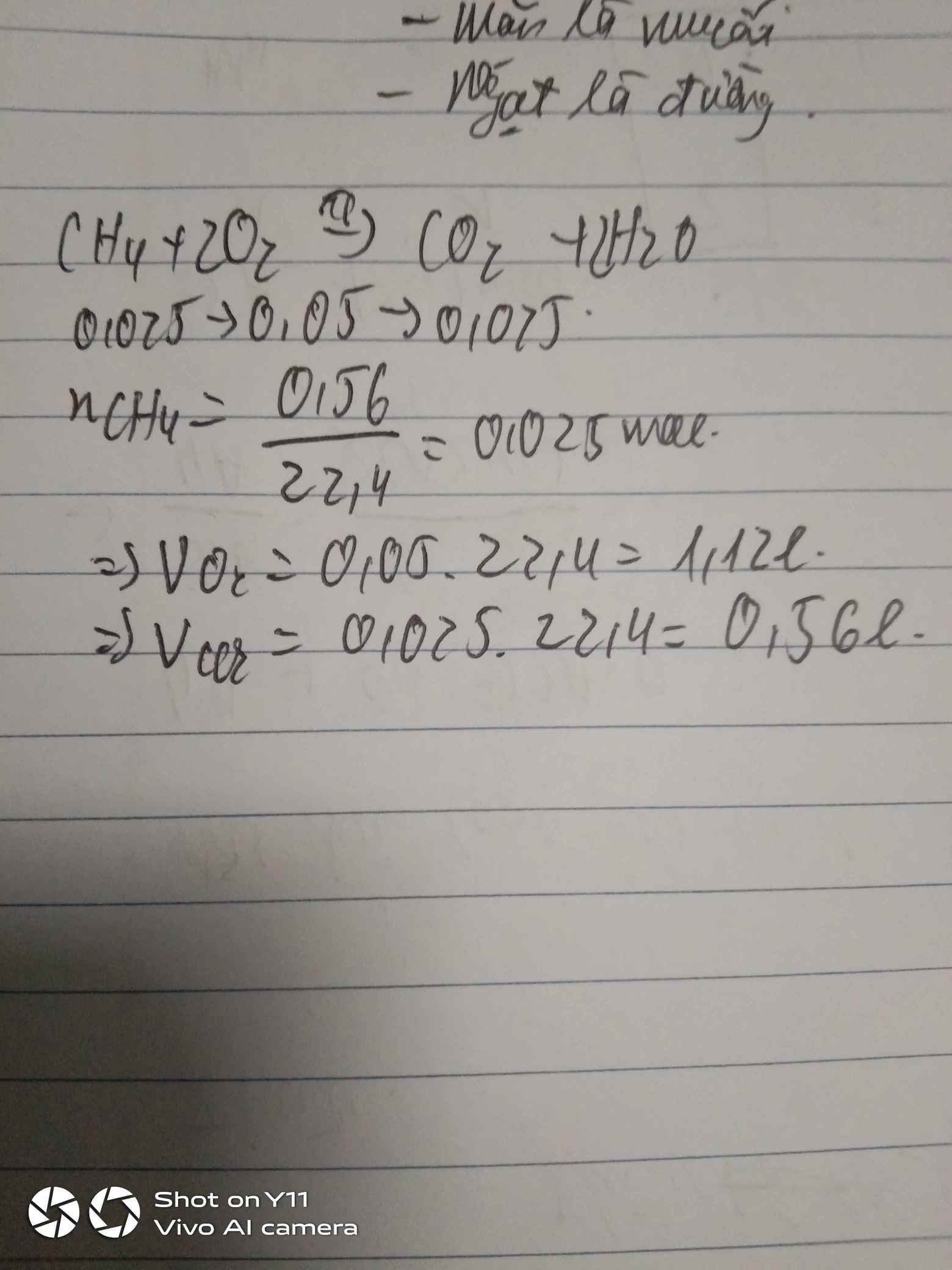a. \(n_{CH_4}=\dfrac{0.56}{22,4}=0,025\left(mol\right)\)
PTHH : CH4 + 2O2 -> CO2 + 2H2O
0,025 0,05 0,025
b. \(V_{O_2}=0,05.22,4=1,12\left(l\right)\)
c. \(V_{CO_2}=0,025.22,4=0,56\left(l\right)\)
nCH4= v/22,4= 0,56/22,4=0,025(mol)
a) CH4+O2-------> CO2+2H20( nhớ ghi điều kiện nhiệt độ)
CH4+ O2-------->CO2+2H2O
1 1 1 2 mol
0,025 0,025 mol
b) VO2=n*22,4=0,025*22,4=0,56(l)