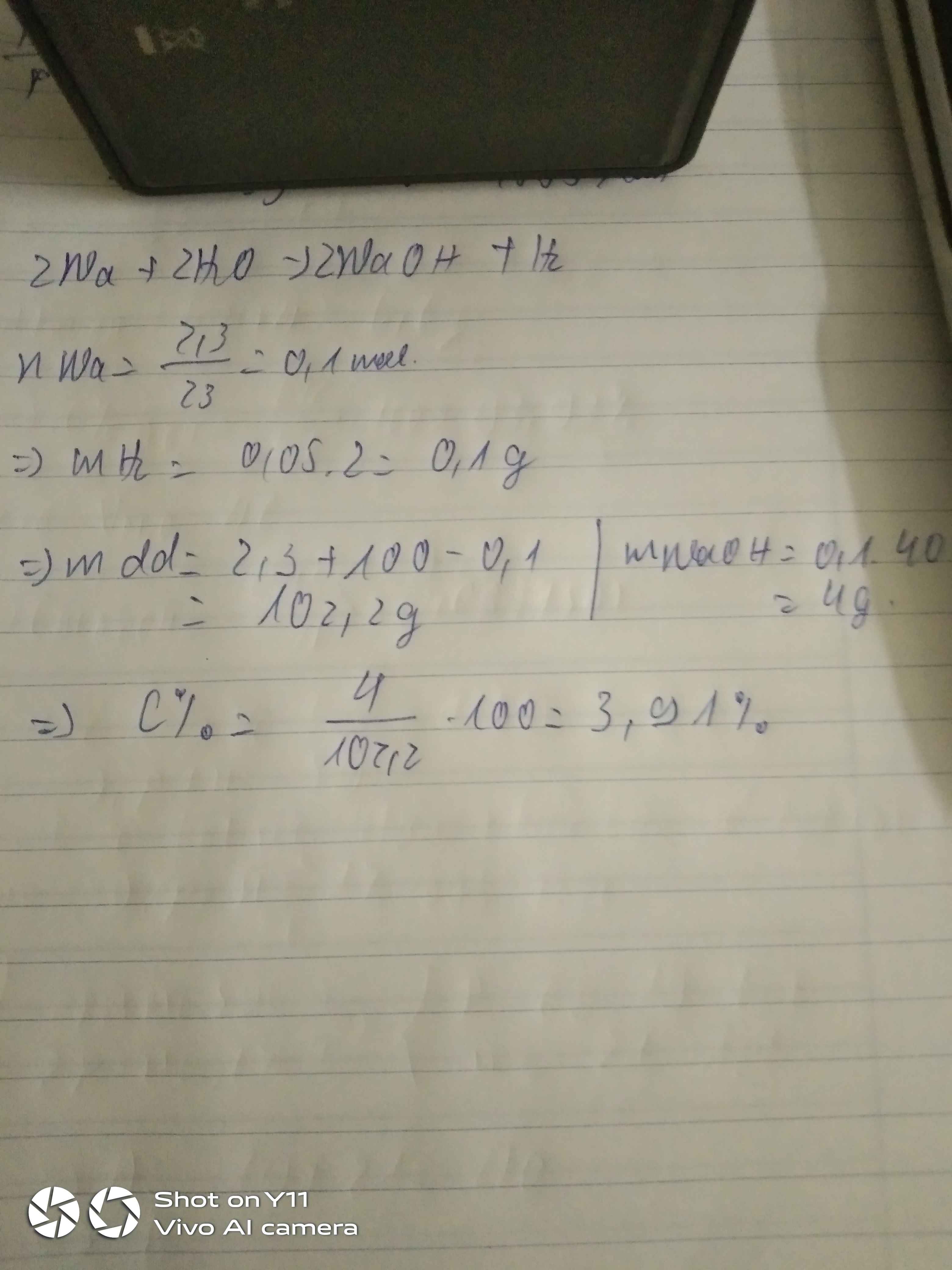\(n_{Na}=\dfrac{2,3}{23}=0,1\left(mol\right)\)
Pt : \(2Na+2H_2O\rightarrow2NaOH+H_2|\)
2 2 2 1
0,2 0,2
\(n_{NaOH}=\dfrac{0,2.2}{2}=0,2\left(mol\right)\)
⇒ \(m_{NaOH}=0,2.40=8\left(g\right)\)
\(m_{ddspu}=2,3+100=102,3\left(g\right)\)
\(C_{ddNaOH}=\dfrac{8.100}{102,3}=7,82\)0/0
Chúc bạn học tốt
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
\(n_{H_2}=\dfrac{1}{2}n_{Na}=\dfrac{1}{2}.\dfrac{2,3}{23}=0,05\left(mol\right)\)
\(\Rightarrow m_{H_2}=0,05.2=0,1\left(g\right)\)
\(\Rightarrow m_{\text{dd sau pư}}=100+2,3-0,1=102,2\left(g\right)\)
\(n_{NaOH}=n_{Na}=0,1\left(mol\right)\)
\(\Rightarrow C\%=\dfrac{0,1.40}{102,2}.100\%=3,91\%\)