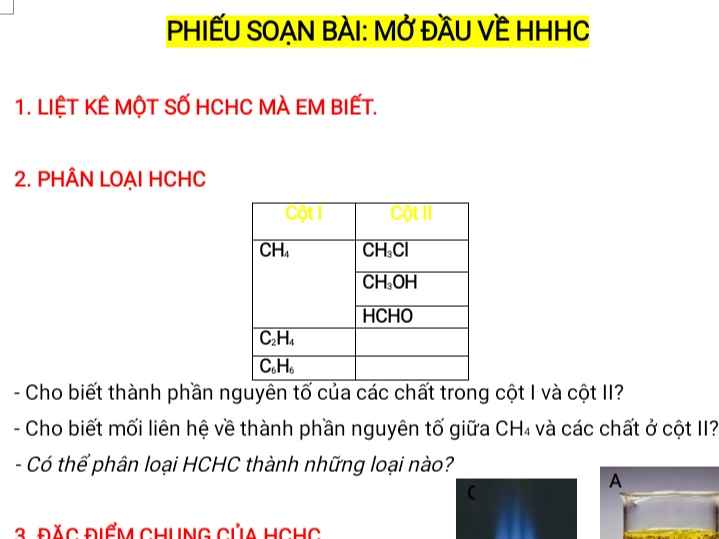
nFe=\(\dfrac{16,8}{56}=0,3mol\)
\(n_{H_2SO_4}=\dfrac{78,4}{98}=0,8mol\)
2Fe+6H2SO4\(\rightarrow\)Fe2(SO4)3+3SO2+6H2O
-Tỉ lệ: \(\dfrac{0,3}{2}=0,15>\dfrac{0,8}{6}\approx0,133\)\(\rightarrow\)Fe dư
\(n_{Fe_2\left(SO_4\right)_3}=\dfrac{1}{6}n_{H_2SO_4}=\dfrac{0,8}{6}=\dfrac{0,4}{3}mol\)
\(n_{Fe\left(pu\right)}=\dfrac{2}{6}n_{H_2SO_4}=\dfrac{2}{6}.0,8=\dfrac{1,6}{6}mol\)
\(n_{Fe\left(dư\right)}=0,3-\dfrac{1,6}{6}=\dfrac{17}{30}mol\)
Fe+Fe2(SO4)3\(\rightarrow\)3FeSO4
-Tỉ lệ: \(\dfrac{1,6}{6}\approx0,26< \dfrac{17}{30}\approx0,56\)\(\rightarrow\)Fe2(SO4)3 dư
Fe+Fe2(SO4)3\(\rightarrow\)3FeSO4
\(\dfrac{1,6}{6}\rightarrow\)\(\dfrac{1,6}{6}\)............0,8
\(n_{Fe_2\left(SO_4\right)_3\left(dư\right)}=\dfrac{17}{30}-\dfrac{1,6}{6}=0,3mol\)
mmuối=\(m_{FeSO_4}+m_{Fe_2\left(SO_4\right)_3}=0,8.152+0,3.400=121,216gam\)