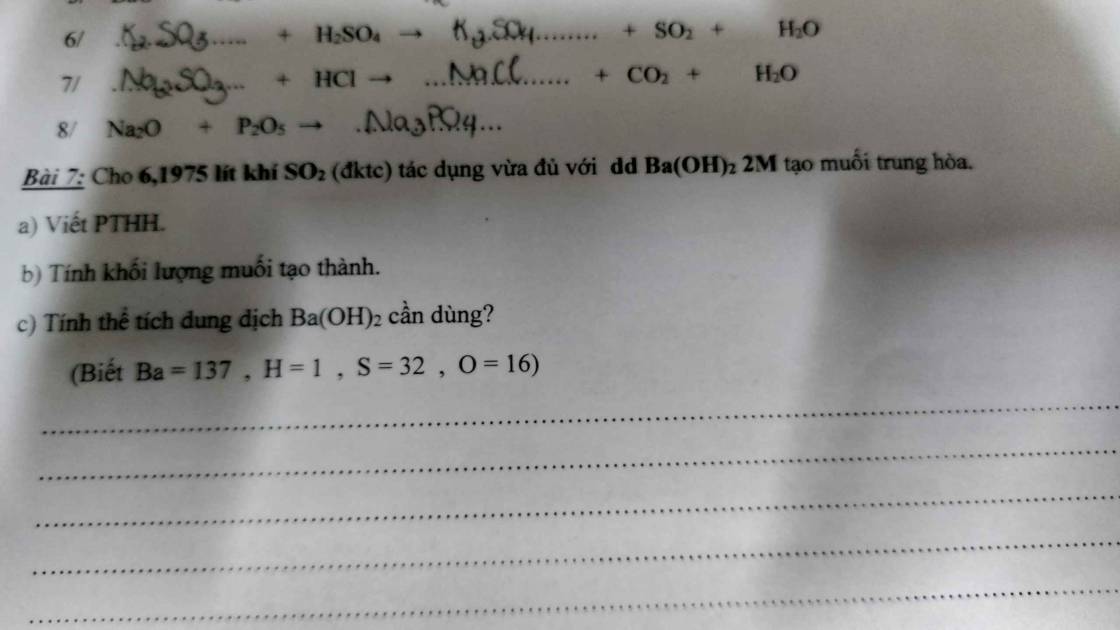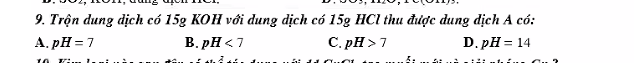Bài 7:
a, PT: \(SO_2+Ba\left(OH\right)_2\rightarrow BaSO_{3\downarrow}+H_2O\)
b, Ta có: \(n_{SO_2}=\dfrac{6,1975}{24,79}=0,25\left(mol\right)\)
Theo PT: \(n_{BaSO_3}=n_{SO_2}=0,25\left(mol\right)\)
\(\Rightarrow m_{BaSO_3}=0,25.217=54,25\left(g\right)\)
c, \(n_{Ba\left(OH\right)_2}=n_{SO_2}=0,25\left(mol\right)\)
\(\Rightarrow V_{Ba\left(OH\right)_2}=\dfrac{0,25}{2}=0,125\left(l\right)\)
Bài 9:
a, A làm quỳ tím hóa xanh.
PT: \(CaO+H_2O\rightarrow Ca\left(OH\right)_2\)
b, \(n_{CaO}=\dfrac{11,2}{56}=0,2\left(mol\right)\)
Theo PT: \(n_{Ca\left(OH\right)_2}=n_{CaO}=0,2\left(mol\right)\)
\(\Rightarrow C_{M_{Ca\left(OH\right)_2}}=\dfrac{0,2}{0,05}=4\left(M\right)\)
c, \(SO_2+Ca\left(OH\right)_2\rightarrow CaSO_{3\downarrow}+H_2O\)
Theo PT: \(n_{SO_2}=n_{Ca\left(OH\right)_2}=0,2\left(mol\right)\Rightarrow V_{SO_2}=0,2.24,79=4,958\left(l\right)\)
d, Ruộng đất bị chua → có tính axit → Rải CaO trên ruộng, CaO gặp nước tạo Ca(OH)2 có tác dụng trung hòa axit.
Bài 8:
a, \(4Al+3O_2\underrightarrow{t^o}2Al_2O_3\)
\(Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\)
b, Ta có: \(n_{Al}=\dfrac{2,7}{27}=0,1\left(mol\right)\)
Theo PT: \(n_{O_2}=\dfrac{3}{4}n_{Al}=0,075\left(mol\right)\Rightarrow V_{O_2}=0,075.24,79=1,85925\left(l\right)\)
c, Theo PT: \(n_{HCl}=6n_{Al_2O_3}=6.\dfrac{1}{2}n_{Al}=0,3\left(mol\right)\)
\(\Rightarrow m_{HCl}=0,3.36,5=10,95\left(g\right)\Rightarrow m_{ddHCl}=\dfrac{10,95}{14,6\%}=75\left(g\right)\)