\(C_6H_5CH_3-2HO-NO_2\underrightarrow{^{H2SO4\left(đ\right)}}C_6H_2\left(CH_3\right)\left(NO_2\right)_3+H_2O\)
Ta có:
\(n_{C6H5CH3}=\frac{23}{92}=0,25\left(mol\right)\)
\(n_{C6H5CH3\left(pư\right)}=0,25.80\%=0,2\left(mol\right)\)
\(n_{C6H5CH3}=n_{C6H5\left(CH3\right)\left(NO2\right)3}=0,2\left(mol\right)\)
\(m_{C6H2\left(CH3\right)\left(NO2\right)3}=0,2.227=45,4\left(g\right)\)






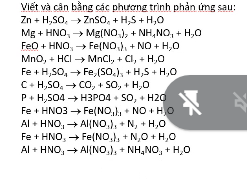 Giúp mình câu này với ạ, mình rối quá
Giúp mình câu này với ạ, mình rối quá


