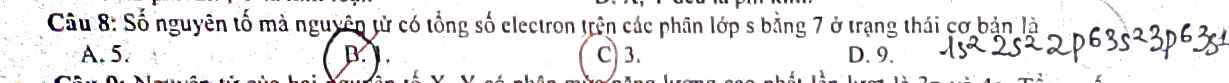Mỗi phần :
n NaCl = a(mol) ; n KBr = b(mol) ; n KI = c(mol)
=> 58,5a + 119b + 166c = 46,25/2 = 23,125(1)
Phần 1 :
2KI + Br2 → 2KBr + I2
c.......................c...........(mol)
=> 58,5a + 119b + 119c = 20,775(2)
Phần 2 :
2KBr + Cl2 → 2KCl + Br2
b.........................b.................(mol)
2KI + Cl2 → 2KCl + I2
c......................c...................(mol)
=> 58,5a + 74,5b + 74,5c = 14,1(3)
(1)(2)(3) => a = 0,05 ; b = 0,1 ; c = 0,05
%m NaCl = 0,05.58,5/23,125 .100% = 12,648%
Đáp án B