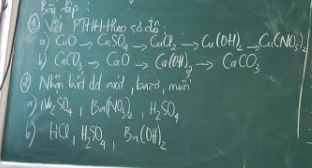a) \(n_{Ba}=\dfrac{13,7}{137}=0,1\left(mol\right)\)
PTHH: Ba + 2H2O --> Ba(OH)2 + H2
0,1-------------->0,1----->0,1
=> VH2 = 0,1.22,4 = 2,24 (l)
b) \(C_{M\left(Ba\left(OH\right)_2\right)}=\dfrac{0,1}{0,2}=0,5M\)
\(n_{Ba}=\dfrac{13,7}{137}=0,1mol\)
\(Ba+2H_2O\rightarrow Ba\left(OH\right)_2+H_2\)
0,1 0,1 0,1 ( mol )
\(V_{H_2}=0,1.22,4=2,24l\)
\(C_{M_{Ba\left(OH\right)_2}}=\dfrac{0,1}{0,2}=0,5M\)











 giải giúp mình câu 5 với ạ
giải giúp mình câu 5 với ạ