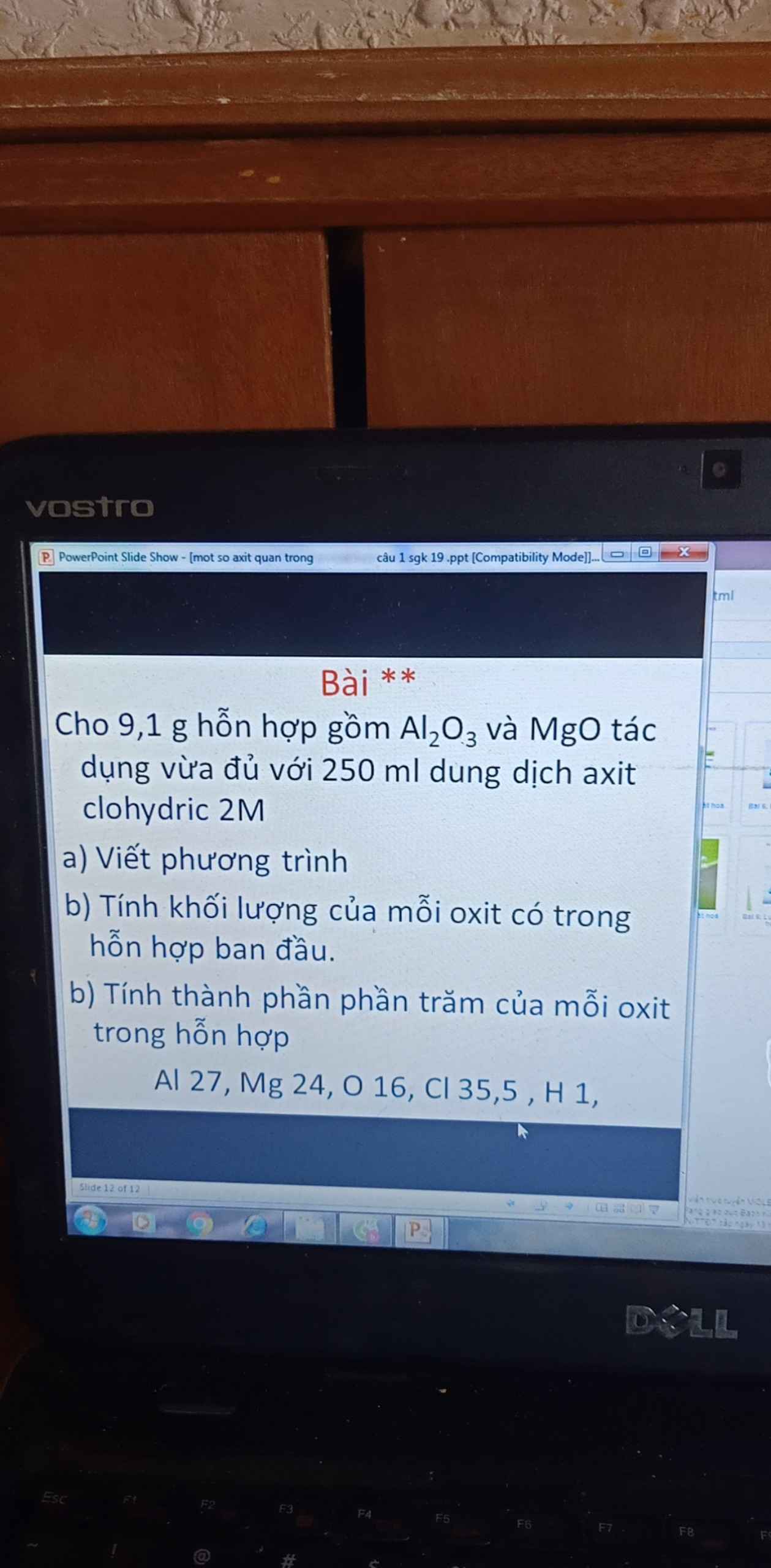
\(n_{HCl}=2.0,25=0,5(mol)\\ a,PTHH:Al_2O_3+6HCl\to 2AlCl_3+3H_2O\\ MgO+2HCl\to MgCl_2+H_2O\)
\(b,\) Đặt \(n_{Al_2O_3}=x(mol);n_{MgO}=y(mol)\)
\(\Rightarrow 102x+40y=9,1(1)\)
Từ 2 PTHH: \(6x+2y=0,5(2)\)
\((1)(2)\Rightarrow x=0,05(mol);y=0,1(mol)\\ \Rightarrow m_{Al_2O_3}=0,05.102=5,1(g)\\\Rightarrow m_{MgO}=9,1-5,1=4(g)\\ c,\%_{Al_2O_3}=\dfrac{5,1}{9,1}.100\%=56,04\%\\ \%_{MgO}=100\%-56,04\%=43,96\%\)
a) MgO + 2HCl --> MgCl2 + H2O
Al2O3 + 6HCl --> 2AlCl3 + 3H2O
b) Gọi số mol MgO, Al2O3 là a,b (mol)
=> 40a + 102b = 9,1
nHCl = 0,25.2=0,5(mol)
PTHH: MgO + 2HCl --> MgCl2 + H2O
_______a------>2a-------->a______________(mol)
Al2O3 + 6HCl --> 2AlCl3 + 3H2O
_b------->6b------>2b__________________(mol)
=> 2a + 6b = 0,5
=> a = 0,1, b = 0,05
c) \(\left\{{}\begin{matrix}\%MgO=\dfrac{0,1.40}{9,1}.100\%=43,96\%\\\%Al_2O_3=\dfrac{0,05.102}{9,1}.100\%=56,04\%\end{matrix}\right.\)