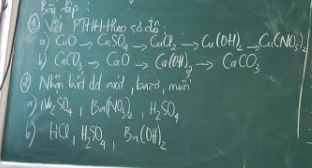\(a,n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\)
PTHH: 2Na + 2H2O ---> 2NaOH + H2
0,2--->0,2--------->0,2------->0,1
=> \(\left\{{}\begin{matrix}a,V=0,1.22,4=2,24\left(l\right)\\b,C_{M\left(NaOH\right)}=\dfrac{0,2}{0,5}=0,4M\\c,m_{H_2O}=0,2.18=3,6\left(g\right)\end{matrix}\right.\)
\(n_{Na}=\dfrac{4,6}{23}=0,2mol\)
\(2Na+2H_2O\rightarrow2NaOH+H_2\)
0,2 0,2 0,2 0,1 ( mol )
\(V_{H_2}=0,1.22,4=2,24l\)
\(C_{M_{NaOH}}=\dfrac{0,2}{0,5}=0,4M\)
\(m_{H_2O}=0,2.18=3,6g\)
\(n_{Na}=\dfrac{4,6}{23}=0,2\left(mol\right)\\
pthh:Na+H_2O\rightarrow NaOH+\dfrac{1}{2}H_2\)
0,2 0,2 0,2 0,1
\(V_{H_2}=0,2.22,4=4,48l\\
C_M=\dfrac{0,2}{0,5}=0,4M\\
m_{H_2O}=0,2.18=3,6g\)










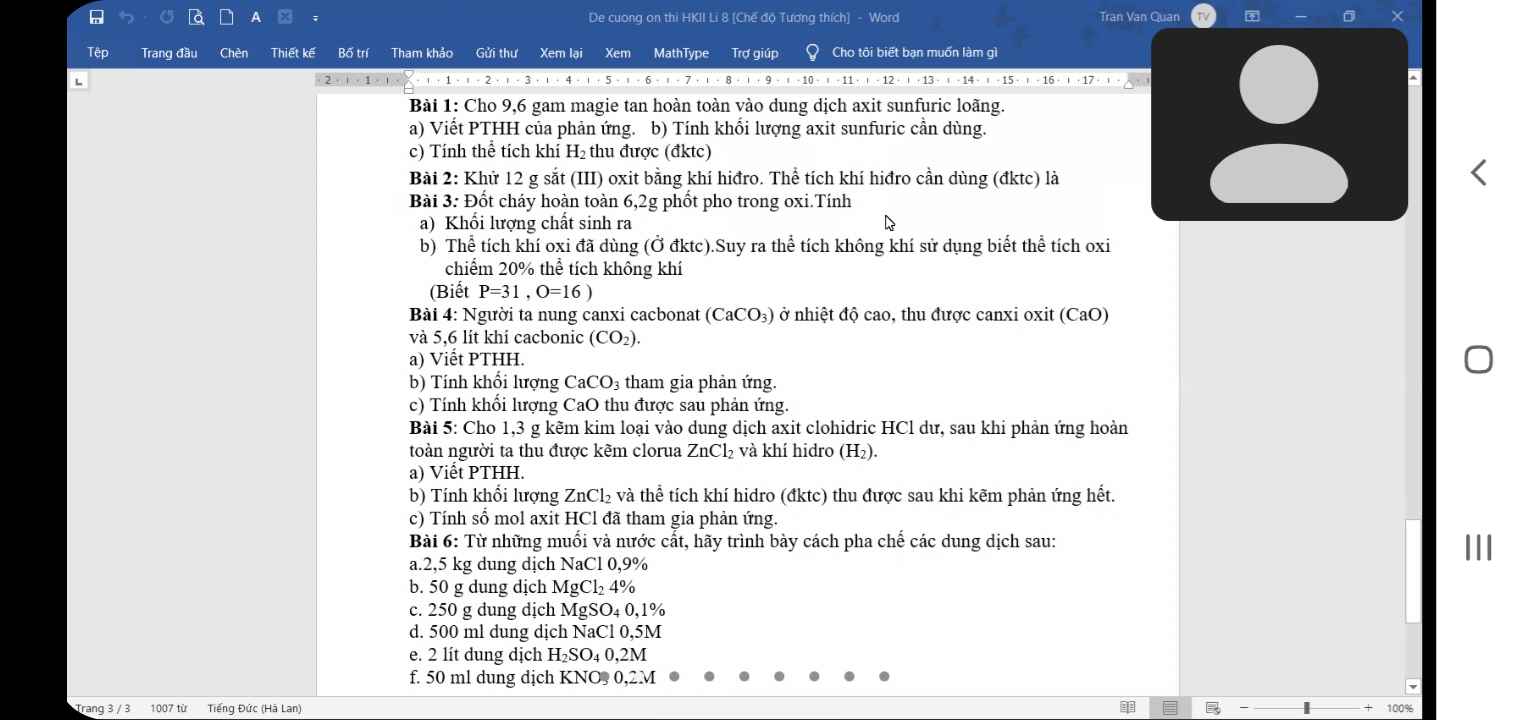
 giải giúp mình câu 5 với ạ
giải giúp mình câu 5 với ạ