\(b,n_{H_2SO_4}=\dfrac{4,9}{98}=0,05\left(mol\right)\\ C_{MddH_2SO_4}=\dfrac{0,05}{0,2}=0,25\left(M\right)\\ c,n_{HCl}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ C_{MddHCl}=\dfrac{0,4}{0,25}=1,6\left(M\right)\\ d,C_{MddHCl}=\dfrac{C\%_{ddHCl}.10.d_{ddHCl}}{M_{HCl}}=\dfrac{7,3.10.1,25}{36,5}=2,5\left(M\right)\)
Chương 1. Sự điện li
Đúng 1
Bình luận (0)
Các câu hỏi tương tự
Làm giúp mình với, mấy câu tính toán giải thích chi tiết ra giùm ạ chứ mình search mạng rồi nhưng không hiểu, làm được hay câu ấy

giải chi tiết dùm em ạ
Đọc tiếp
giải chi tiết dùm em ạ





Giải chi tiết bài 30 giúp mik vs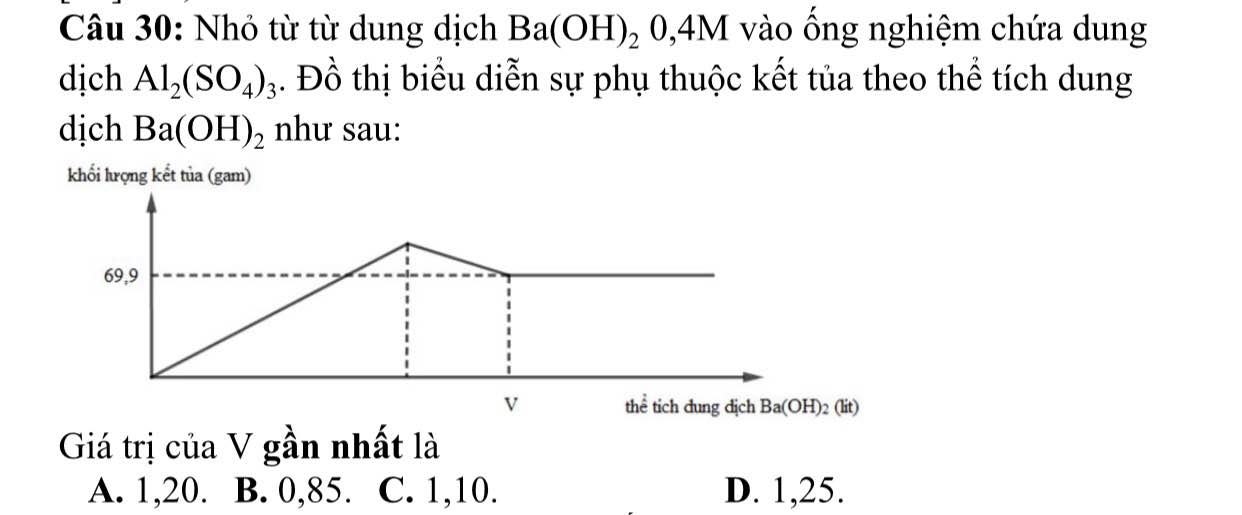
Cân bằng pư oxi hóa khử ( giải chi tiết ) M + HNO3 = M(NO3)3 + NO + H2O CxHy + H2SO4= SO2+ CO2+ H20
Cho 200 ml dung dịch X gồm NaOH 0,2M và Ba(OH)2 0,3M. Thể tích dung dịch H2SO4 0,5M cần để trung hòa hết dung dịch X là bao nhiêu?
mọi người giúp mk vs. thanks
cố gắng giải chi tiết dùm mk vs.
Câu 7. Các dung dịch sau có môi trường gì? Giải thích.AlCl3, (CH3COO)2Ba, KNO3, K2S, NH4NO3, NaNO2. mọi người giúp với ạ cảm ơn mn nhiều
Pha loãng 1 lít dd NaOH có pH = 13 bằng bao nhiêu lít nước để được dd mới có pH = 12 ?
A. 90
B. 9
C. 10
D. 100
Trình bày bài giải chi tiết rồi mới chọn đáp án nha các bạn .
HELP ME !!!!!!
Hòa tan 0,78 gam một kim loại kiềm vào 2 lít nước được dung dịch có pH = 12 . Kim loại đó là :
A. Cs
B. Li
C. Na
D. K
Trình bày bài giải chi tiết rồi ms chọn đáp án nha các bạn .
HELP ME !!!
Thể tích dung dịch HCl 0,03M cần để trung hòa 100 ml dung dịch hỗn hợp gồm NaOH 0,1M và Ba(OH)2 0,1M là :
A. 200 ml
B. 150 ml
C. 250 ml
D. 100 ml
Trình bày bài giải chi tiết rồi mới chọn đáp án nha các bạn .
HELP ME !!!!












