\(CH_3COOH+C_2H_5OH⇌\left(H^+,t^o\right)CH_3COOC_2H_5+H_2O\\ n_{CH_3COOH}=\dfrac{12}{60}=0,2\left(mol\right);n_{C_2H_5OH}=\dfrac{13,8}{46}=0,3\left(mol\right)\\ Vì:\dfrac{0,3}{1}>\dfrac{0,2}{1}\Rightarrow C_2H_5OH.dư\\ n_{CH_3COOC_2H_5\left(LT\right)}=n_{CH_3COOH}=0,2\left(mol\right)\\ n_{CH_3COOC_2H_5\left(TT\right)}=\dfrac{11}{88}=0,125\left(mol\right)\\ \Rightarrow H=\dfrac{0,125}{0,2}.100\%=62,5\%\)
Bài 19: Carboxylic acid
Đúng 2
Bình luận (0)
Các câu hỏi tương tự
Do phản ứng ester hoá là phản ứng thuận nghịch nên hiệu suất của phản ứng thường không cao. Đề xuất các biện pháp để nâng cao hiệu suất của phản ứng ester hoá.
Trình bày phương pháp hoá học để phân biệt các dung dịch sau: ethanol, glycerol, acetaldehyde và acetic acid.
Chuẩn bị: Cồn 96°, acetic acid nguyên chất, dung dịch H2SO4 đặc, dung dịch NaCl bão hoà, ống nghiệm.Tiến hành: Cho 1 mL cồn 96° vào trong ống nghiệm. Cho tiếp vào trong ống nghiệm 1 mL acetic acid nguyên chất. Thêm vào ống nghiệm 1 – 2 giọt dung dịch sulfuric acid đậm đặc và lắc đều, dùng bông sạch nút miệng ống nghiệm. Sau đó, đun cách thuỷ trong cốc thuỷ tinh ở nhiệt độ 65 – 70 °C trong khoảng thời gian 5 – 7 phút. Làm lạnh ống nghiệm rồi cho thêm vào 2 mL dung dịch sodium chloride bão hoà. Để...
Đọc tiếp
Chuẩn bị: Cồn 96°, acetic acid nguyên chất, dung dịch H2SO4 đặc, dung dịch NaCl bão hoà, ống nghiệm.
Tiến hành: Cho 1 mL cồn 96° vào trong ống nghiệm. Cho tiếp vào trong ống nghiệm 1 mL acetic acid nguyên chất. Thêm vào ống nghiệm 1 – 2 giọt dung dịch sulfuric acid đậm đặc và lắc đều, dùng bông sạch nút miệng ống nghiệm. Sau đó, đun cách thuỷ trong cốc thuỷ tinh ở nhiệt độ 65 – 70 °C trong khoảng thời gian 5 – 7 phút. Làm lạnh ống nghiệm rồi cho thêm vào 2 mL dung dịch sodium chloride bão hoà. Để yên ống nghiệm.
Yêu cầu: Quan sát, mô tả hiện tượng và giải thích.
Chú ý an toàn: Cẩn thận khi làm việc với dung dịch H2SO4 đặc.
Hãy viết công thức cấu tạo của acetic acid. Cho biết một số tính chất hoá học và ứng dụng của acetic acid mà em biết.
Chuẩn bị: Dung dịch acetic acid 5%, giấy quỳ tím, đũa thủy tinh.
Tiến hành: Nhúng đầu đũa thuỷ tinh vào dung dịch acetic acid 5%, sau đó chấm vào giấy quỳ tím. Quan sát và nhận xét sự thay đổi màu của giấy quỳ tím.
Yêu cầu: Quan sát và nhận xét sự thay đổi màu của giấy quỳ tím.
Trình bày cách phân biệt các dung dịch sau: acetic acid, acrylic acid, acetaldehyde.
Giấm được sử dụng khá phổ biến để chế biến thức ăn. Bạn Mai muốn xác định nồng độ acetic acid có trong giấm ăn bằng cách sử dụng dung dịch sodium hydroxyde 0,1M để chuẩn độ. Bạn lấy mẫu giấm ăn đó để làm thí nghiệm và kết quả chuẩn độ 3 lần như bảng sau:Hãy giúp bạn Mai xác định nồng độ mol của acetic acid trong giấm
Đọc tiếp
Giấm được sử dụng khá phổ biến để chế biến thức ăn. Bạn Mai muốn xác định nồng độ acetic acid có trong giấm ăn bằng cách sử dụng dung dịch sodium hydroxyde 0,1M để chuẩn độ. Bạn lấy mẫu giấm ăn đó để làm thí nghiệm và kết quả chuẩn độ 3 lần như bảng sau:
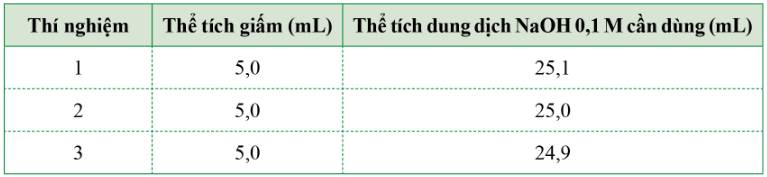
Hãy giúp bạn Mai xác định nồng độ mol của acetic acid trong giấm
Chuẩn bị: Dung dịch CH3COOH 1 M, dung dịch Na2CO3 1M; ống nghiệm, diêm.
Tiến hành: Cho 1 – 2 mL dung dịch sodium carbonate 1 M vào ống nghiệm. Nhỏ tiếp vào ống nghiệm 1 – 2 mL dung dịch acetic acid 1 M. Đưa que diêm đang cháy vào miệng ống nghiệm.
Yêu cầu: Quan sát, mô tả hiện tượng xảy ra và giải thích.
Chuẩn bị: Dung dịch CH3COOH 1 M, phoi bào magnesium; ống nghiệm.
Tiến hành: Cho 1 – 2 mL dung dịch acetic acid 1M vào ống nghiệm, sau đó thêm vào vài mẩu magnesium.
Yêu cầu: Quan sát, mô tả hiện tượng xảy ra và giải thích.



