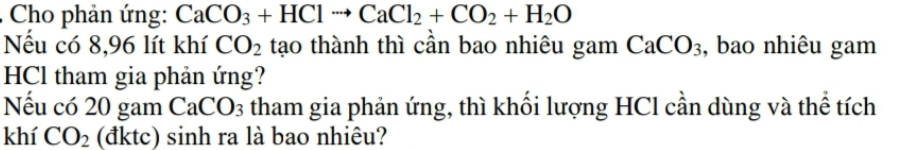\(n_{CO_2}=\dfrac{8,96}{22,4}=0,4\left(mol\right)\\ CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\\ n_{HCl}=2.0,4=0,8\left(mol\right)\\ m_{HCl}=0,8.36,5=29,2\left(g\right)\\ \text{Ý}.sau:CaCO_3+2HCl\rightarrow CaCl_2+CO_2+H_2O\\ n_{CaCO_3}=\dfrac{20}{100}=0,2\left(mol\right)\\ n_{CO_2}=n_{CaCO_3}=0,2\left(mol\right)\\ V_{CO_2\left(\text{đ}ktc\right)}=0,2.22,4=4,48\left(l\right)\)
CHƯƠNG II: PHẢN ỨNG HÓA HỌC
Đúng 2
Bình luận (0)
Các câu hỏi tương tự
1,Cho 2,8 gam sắt vào dung dịch axit clohidric thu được muối sắt (II) clorua và 1 khí hỏi khí đó là khí gì? tính thể tích? tính khối lượng axit đã phản ứng?
2, cần nhiệt phân bao nhiêu g CaCO3 để đc 42g CaO?
mong mn ghi rõ cách giải, cảm ơn nhiều :)
1 ) Trong phản ứng hóa học cho biết : a, hạt vi mô nào được bảo toàn , hạt nào có thể bị chia nhỏ ra ? b, Nguyên tử có bị chia nhỏ không ? c, vì sao có sự biến đổi từ phân tử này sang phân tử khác ? vì sao có sự biến đổi chất này thành chất khác trong phản ứng hóa học ?
thầy phynit ơi sao em thấy môn hóa ít được tick vậy?
em thấy các bạn bên môn Anh hầu như cái nào cũng được tick
như vậy không công bằng cho tất cả các bạn
với lại em thấy nhiều câu làm đúng thầy k tick ạ
em cảm ơn!
Hòa tan a gam đồng(II) oxit vào dung dịch chứa 98g axit sunfuric ( có dư 40% ) thì sau phản ứng thu được b gam đồng (II) sunfat và 10,8g nước
Câu hỏi: Hãy tính giá trị a và b, biết a : b= 1: 2
Giải hộ mình với! Cảm ơn rất nhiều !
Kể tên 15 hiện tượng hóa học (không có trong SGK, SBT). Cảm ơn trước ạ
Mấy bạn ơi, cho mình hỏi làm sao để cân bằng một phương trình theo hướng x,y vậy? Mình suy nghĩ mãi là ko ra? Ví dụ như
\(C_xH_yO_z+O_2\rightarrow CO_2+H_2O\)
Mấy bạn ghi xong đáp án rồi giải thích cho mình với nha. Cảm ơn nhiều ạ!
Nhờ anh chị giúp e với ạ e cảm ơn nhiều

Khi than cháy trong không khí xảy ra phản ứng giữa than (cacbon) và khí oxi
a. Giải thích vì sao cần đập vừa nhỏ than trước khi đưa vào lò, sau đó dùng que lửa châm rồi quạt mạnh đến khi than cháy bén thì thôi?
b. Cho biết phản ứng này có lợi cho môi trường không? Vì sao?
-GIÚP MIK VS AK!
Hòa tan a gam Al và b gam Zn vào dung dịch axit H2SO4 dư thu được những thể tích khí H2 bằng nhau. Tính tỉ lệ a:b
Hãy cứu lấy tui~
Cảm ơn nhiềuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuu~