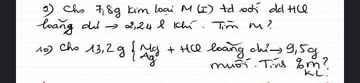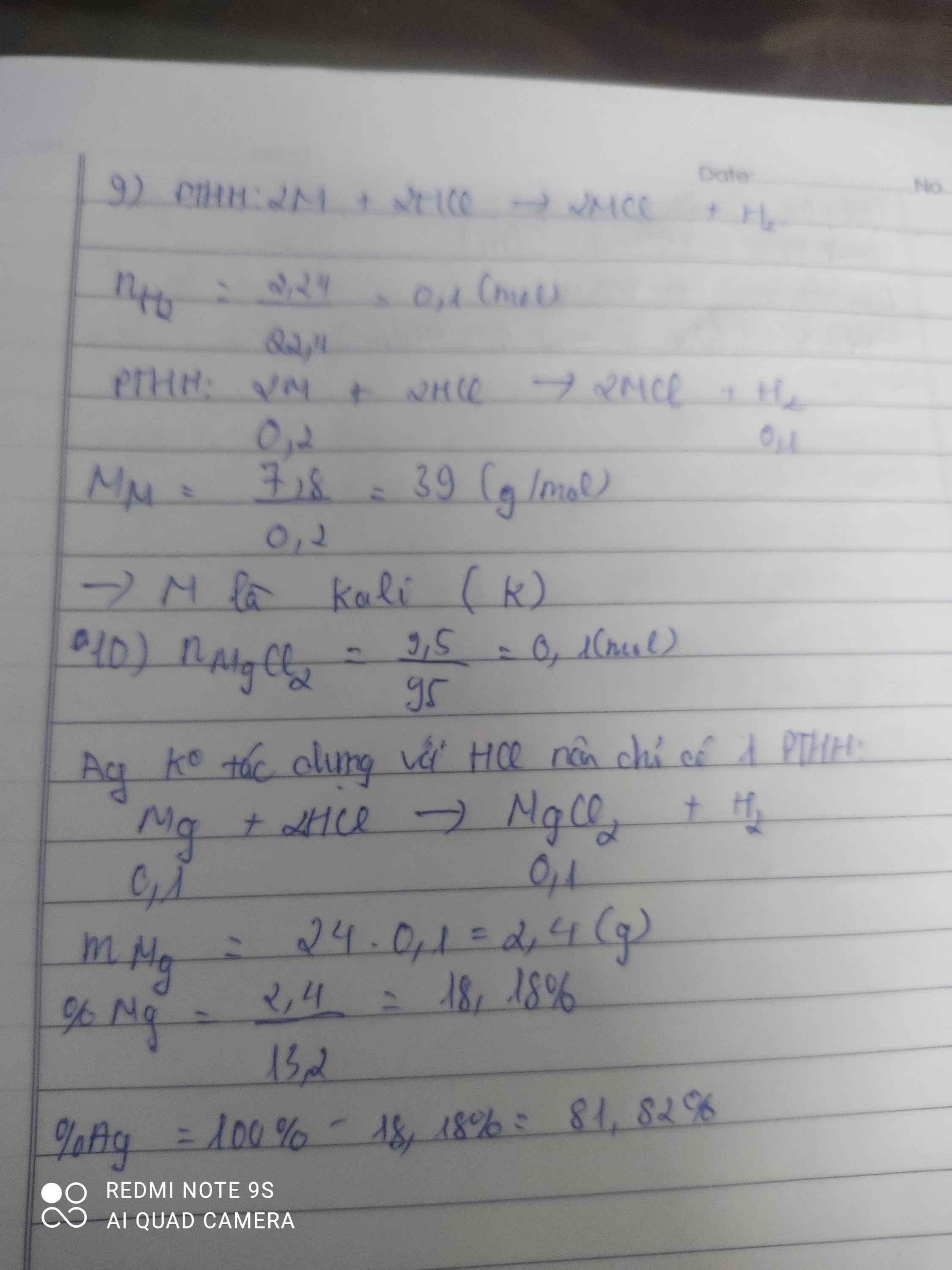1. Cho 18,4 gam hỗn hợp Al và Zn tác dụng với dd HCl dư thấy có 1 gam khí hidro thoát ra. Xác định thành phần % khối lượng mỗi kim loại trong hỗn hợp đầu.
2. Cho hỗn hợp gồm Fe và FeO vào dd HCl¬¬ dư, sau phản ứng thấy có 2,24 lít khí (đktc) thoát ra, cô cạn dung dịch sau phản ứng thu được 38,1 gam muối khan. Tính khối lượng mỗi chất trong hỗn hợp ban đầu.
3. Hòa tan hoàn toàn 20 gam hỗn hợp Zn và Cu bằng lượng vừa đủ dd HCl 2M thu được 4,48 lít khí (đktc). a. Tính th...
Đọc tiếp
1. Cho 18,4 gam hỗn hợp Al và Zn tác dụng với dd HCl dư thấy có 1 gam khí hidro thoát ra. Xác định thành phần % khối lượng mỗi kim loại trong hỗn hợp đầu.
2. Cho hỗn hợp gồm Fe và FeO vào dd HCl¬¬ dư, sau phản ứng thấy có 2,24 lít khí (đktc) thoát ra, cô cạn dung dịch sau phản ứng thu được 38,1 gam muối khan. Tính khối lượng mỗi chất trong hỗn hợp ban đầu.
3. Hòa tan hoàn toàn 20 gam hỗn hợp Zn và Cu bằng lượng vừa đủ dd HCl 2M thu được 4,48 lít khí (đktc). a. Tính thành phần % khối lượng mỗi kim loại trong hỗn hợp ban đầu. b. Tính thể tích dd HCl đã dùng.
4. Cho 22 gam hỗn hợp Fe và Al tác dụng vừa đủ với dung dịch HCl 7,3%. Sau phản ứng thu được 17,92 lít khí (đktc). a. Tính thành phần % khối lượng mỗi kim loại trong hỗn hợp ban đầu. b. Tính khối lượng dung dịch HCl đã dùng.
5*. Cho m gam hỗn hợp 2 kim loại kiềm (ở 2 chu kỳ liên tiếp nhau) tác dụng vừa đủ với dung dịch HCl thu được 0,448 lít khí (đktc). Dung dịch thu được sau phản ứng đem cô cạn được 2,58 gam muối khan. a. Xác định tên 2 kim loại. b. Tính thành phần % khối lượng mỗi kim loại trong hỗn hợp ban đầu.
6*. Chia 35 gam hỗn hợp X chứa Fe, Cu, Al thành 2 phần bằng nhau. - Phần 1: cho tác dụng hoàn toàn với dung dịch HCl dư thu được 6,72 lít khí (đktc). - Phần 2: tác dụng vừa đủ với 10,64 lít khí clo (đktc). Tính thành phần % khối lượng mỗi kim loại trong hỗn hợp ban đầu.