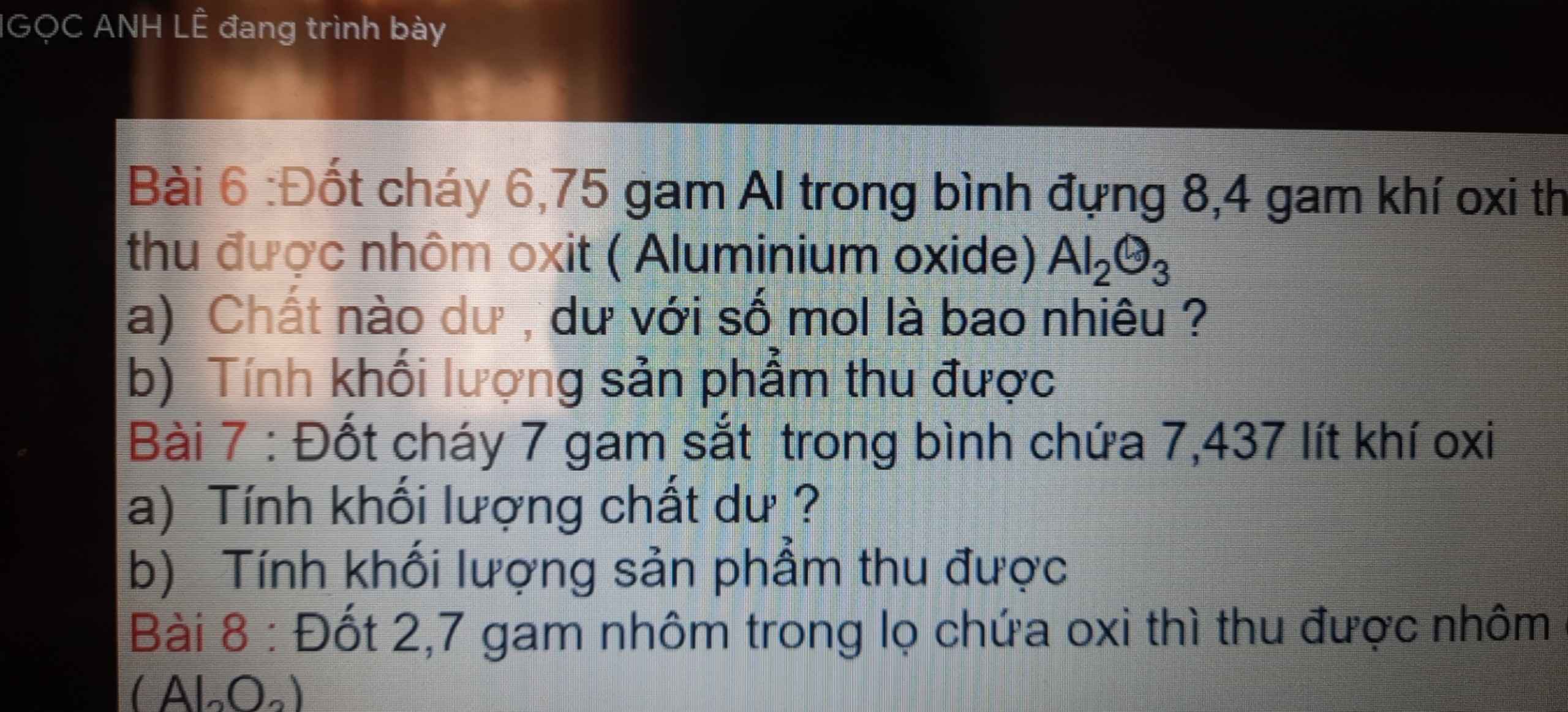
Bài 6:
\(a,n_{Al}=\dfrac{6,75}{27}=0,25\left(mol\right)\\ n_{O_2}=\dfrac{8,4}{32}=0,2625\left(mol\right)\\ 4Al+3O_2\rightarrow\left(t^o\right)2Al_2O_3\\ Vì:\dfrac{0,25}{4}< \dfrac{0,2625}{3}\Rightarrow O_2dư\\ n_{O_2\left(dư\right)}=0,2625-\dfrac{3}{4}.0,25=0,075\left(mol\right)\\ b,n_{Al_2O_3}=\dfrac{2}{4}.n_{Al}=\dfrac{2}{4}.0,25=0,125\left(mol\right)\\ \Rightarrow m_{Al_2O_3}=102.0,125=12,75\left(g\right)\)
Bài 7:
\(a,n_{Fe}=\dfrac{7}{56}=0,125\left(mol\right)\\ n_{O_2}=\dfrac{7,437}{24,79}=0,3\left(mol\right)\\ 3Fe+2O_2\rightarrow\left(t^o\right)Fe_3O_4\\ Vì:\dfrac{0,125}{3}< \dfrac{0,3}{2}\Rightarrow O_2dư\\ b,n_{Fe_3O_4}=\dfrac{1}{3}.n_{Fe}=\dfrac{0,125}{3}=\dfrac{1}{24}\left(mol\right)\\ \Rightarrow m_{sp}=m_{Fe_3O_4}=\dfrac{232}{24}=\dfrac{29}{3}\left(g\right)\)