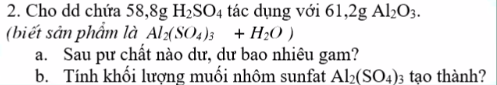a) \(n_{H_2SO_4}=\dfrac{58,8}{98}=0,6\left(mol\right)\); \(n_{Al_2O_3}=\dfrac{61,2}{102}=0,6\left(mol\right)\)
PTHH: Al2O3 + 3H2SO4 --> Al2(SO4)3 + 3H2O
Xét tỉ lệ: \(\dfrac{0,6}{1}>\dfrac{0,6}{3}\) => H2SO4 hết, Al2O3 dư
b) PTHH: Al2O3 + 3H2SO4 --> Al2(SO4)3 + 3H2O
_______________0,6---------->0,2______________(mol)
=> \(m_{Al_2\left(SO_4\right)_3}=0,2.342=68,4\left(g\right)\)