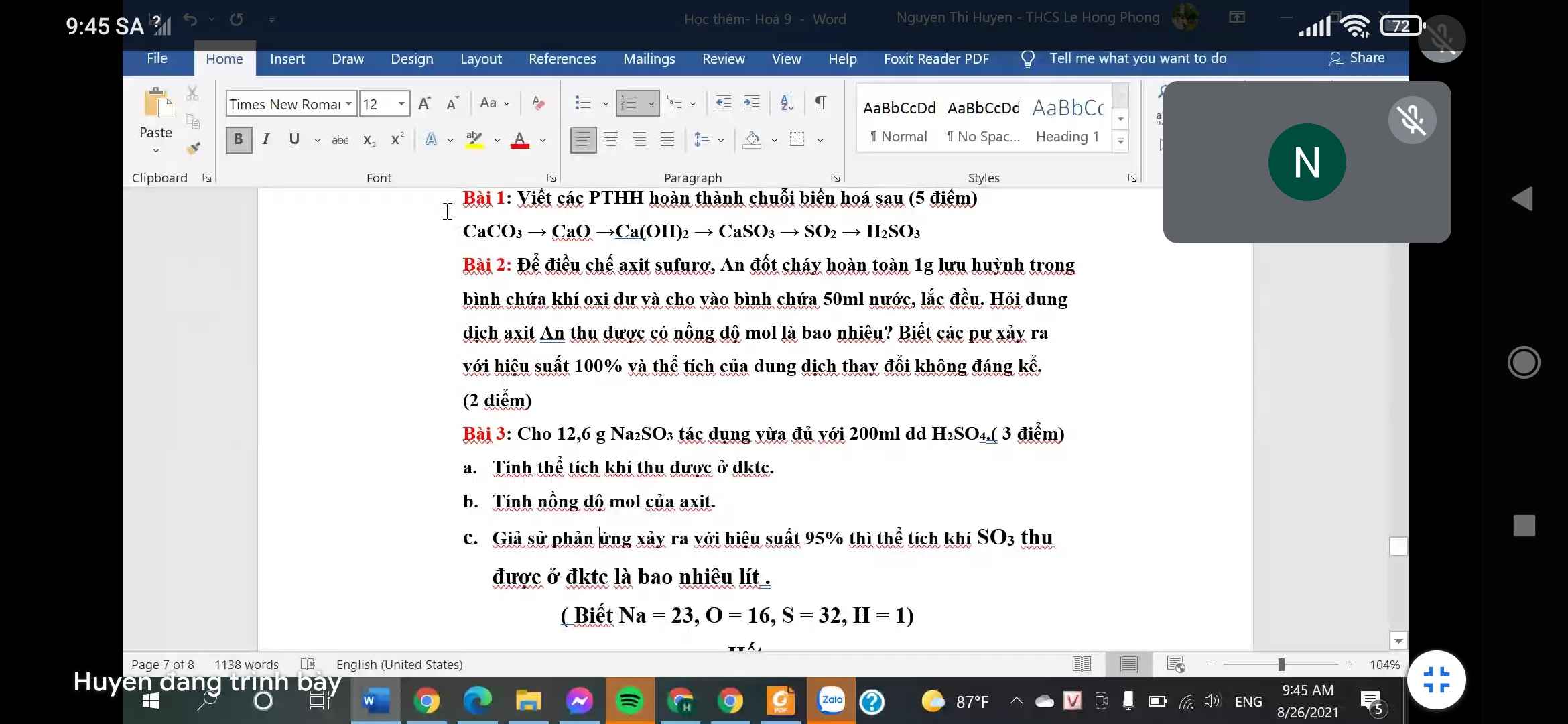
Bài 2 :
\(n_S=\dfrac{1}{32}\left(mol\right)\)
\(S\rightarrow SO_2\rightarrow SO_3\rightarrow H_2SO_4\)
\(\Rightarrow n_{H_2SO_4}=n_S=\dfrac{1}{32}\left(mol\right)\)
\(C_{M_{H_2SO_4}}=\dfrac{\dfrac{1}{32}}{0.05}=0.625\left(M\right)\)
Bài 3 :
\(n_{Na_2SO_3}=\dfrac{12.6}{126}=0.1\left(mol\right)\)
\(Na_2SO_3+H_2SO_4\rightarrow Na_2SO_4+SO_2+H_2O\)
\(0.1...............0.1............................0.1\)
\(a.\)
\(V_{SO_2}=0.1\cdot22.4=2.24\left(l\right)\)
\(b.\)
\(C_{M_{H_2SO_4}}=\dfrac{0.1}{0.2}=0.5\left(M\right)\)
\(c.\)
\(V_{SO_2}=2.24\cdot95\%=2.128\left(l\right)\)
Bài 1 :
\(CaCO_3\underrightarrow{^{^{t^0}}}CaO+CO_2\)
\(CaO+H_2O\rightarrow Ca\left(OH\right)_2\)
\(Ca\left(OH\right)_2+SO_2\rightarrow CaSO_3+H_2O\)
\(CaSO_3+2HCl\rightarrow CaCl_2+SO_2+H_2O\)
\(SO_2+H_2O⇌H_2SO_3\)