cứu emmm
cứu emmm

Những câu hỏi liên quan
cứu emmm
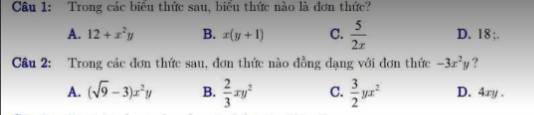
Cứu emmm

\(\lim\limits_{x\rightarrow-\infty}\dfrac{\left|x\right|+\sqrt{x^2+x}}{x+10}\)
\(=\lim\limits_{x\rightarrow-\infty}\dfrac{-x+\sqrt{x^2+x}}{x+10}\)
\(=\lim\limits_{x\rightarrow-\infty}\dfrac{-1+\sqrt{1+\dfrac{1}{x}}}{1+\dfrac{10}{x}}=\dfrac{-1+\sqrt{1}}{1}=\dfrac{-1+1}{1}=0\)
Đúng 1
Bình luận (0)
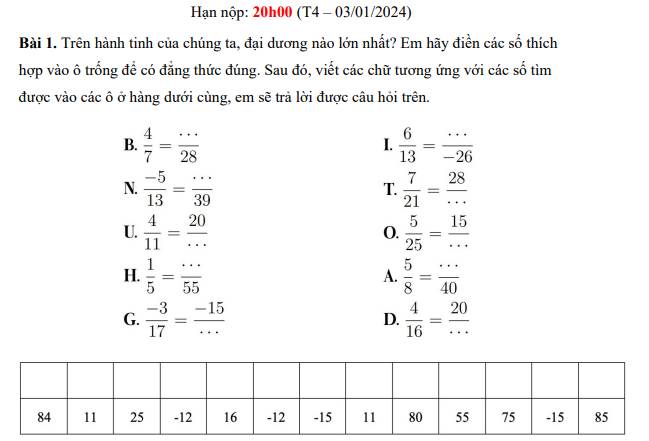 Gấp lắm r aaa, speed run 8h hnay nộp bài r cứu emmm, mãi iuu
Gấp lắm r aaa, speed run 8h hnay nộp bài r cứu emmm, mãi iuu
B = \(\dfrac{4}{7}\) = \(\dfrac{4.4}{7.4}\) = \(\dfrac{16}{28}\); I = \(\dfrac{6}{13}\) = \(\dfrac{6.\left(-2\right)}{13.\left(-2\right)}\) = \(\dfrac{-12}{-26}\)
N = \(\dfrac{-5}{13}\) = \(\dfrac{-5.3}{13.3}\) = \(\dfrac{-15}{39}\); T = \(\dfrac{7}{21}\) = \(\dfrac{7.4}{21.4}\) = \(\dfrac{28}{84}\)
U = \(\dfrac{4}{11}\) = \(\dfrac{4.5}{11.5}\) = \(\dfrac{20}{55}\); O = \(\dfrac{5}{25}\) = \(\dfrac{5.3}{25.3}\) = \(\dfrac{15}{75}\)
H = \(\dfrac{1}{5}\) = \(\dfrac{1.11}{5.11}\) = \(\dfrac{11}{15}\); A = \(\dfrac{5}{8}\) = \(\dfrac{5.5}{8.5}\) = \(\dfrac{25}{40}\)
G = \(\dfrac{-3}{17}\) = \(\dfrac{-3.5}{17.5}\) = \(\dfrac{-15}{85}\); D = \(\dfrac{4}{16}\) = \(\dfrac{4.5}{16.5}\) = \(\dfrac{20}{80}\)
| T | H | A | I | B | I | N | H | D | U | O | N | G |
| 84 | 11 | 25 | -12 | 16 | -12 | -15 | 11 | 80 | 55 | 75 | -15 | 85 |
Đúng 0
Bình luận (0)
cứu emmm
Câu 58. Đun nóng hỗn hợp gồm 6(g) rượu etylic và 6(g) axit axetic có H2SO4 đặc, Nếu hiệu suất phản ứng đạt 75% thì khối lượng este tạo thành là?
A. 7,2(g) B. 8,6(g) C. 6,6(g) D. 8,8(g)
giúp emmm
Giúp emmm
helpp emmm
Bài 1 :
\(2H_2 + O_2 \xrightarrow{t^o} 2H_2O\\ S + O_2 \xrightarrow{t^o} SO_2\\ 4Al + 3O_2 \xrightarrow{t^o} 2Al_2O_3\\ 4P + 5O_2 \xrightarrow{t^o} 2P_2O_5\)
Đúng 1
Bình luận (0)
Bài 2 :
\(3Fe + 2O_2 \xrightarrow{t^o} Fe_3O_4\\ a) n_{Fe_3O_4} = \dfrac{2,32}{232} = 0,01(mol)\\ n_{Fe} = 3n_{Fe_3O_4} = 0,03(mol)\Rightarrow m_{Fe} = 0,03.56 = 1,68(gam)\\ n_{O_2} = 2n_{Fe_3O_4} = 0,02(mol) \Rightarrow m_{O_2} = 0,02.32 = 0,64(gam)\\ b) 2KMnO_4 \xrightarrow{t^o} K_2MnO_4 + MnO_2 + O_2\\ n_{KMnO_4} = 2n_{O_2} = 0,04(mol)\Rightarrow m_{KMnO_4} = 0,04.158= 6,32(gam)\)
Đúng 1
Bình luận (0)
Giúp emmm
Cứuu emmm

Giúp emmm:((