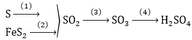1 tấn = 1000 kg
$m_{FeS_2} = 1000.80\% = 800(kg)$
$n_{FeS_2} = \dfrac{800}{120} = \dfrac{20}{3}(kmol)$
Bảo toàn S :
$n_{H_2SO_4} = 2n_{FeS_2} = \dfrac{40}{3}(kmol)$
$m_{H_2SO_4} = \dfrac{40}{3}.98 = 1306,67(kg)$
$m_{H_2SO_4\ thu\ được} = 1306,67.95\% = 1241,3(kg)$
\(m_{FeS_2}=\dfrac{1.80}{100}=0,8\left(tấn\right)\)
=> \(n_{FeS_2}=\dfrac{0,8.10^6}{120}=\dfrac{20000}{3}\left(mol\right)\)
Bảo toàn S: \(n_{H_2SO_4\left(LT\right)}=\dfrac{40000}{3}\left(mol\right)\)
=> \(n_{H_2SO_4\left(TT\right)}=\dfrac{40000}{3}.95\%=\dfrac{38000}{3}\left(mol\right)\)
=> \(m_{H_2SO_4\left(TT\right)}=\dfrac{38000}{3}.98=\dfrac{931}{750}.10^6\left(g\right)=\dfrac{931}{750}\left(tấn\right)\)
=> \(m_{dd.H_2SO_4.60\%}=\dfrac{\dfrac{931}{750}.100}{60}=\dfrac{931}{450}\left(tấn\right)\)