| Chất | M(g/mol) | n(mol) | m(g) | V(lít) (đkc) |
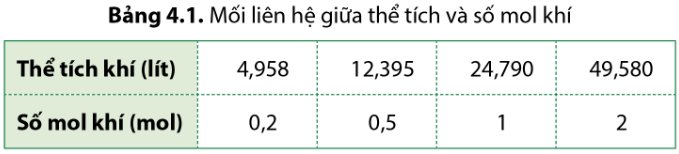
| CO2 | \(M_{CO_2}=M_C+2.M_O=12+2.16=44\left(\dfrac{g}{mol}\right)\) | \(n_{CO_2}=\dfrac{m_{CO_2}}{M_{CO_2}}=\dfrac{17,6}{44}=0,4\left(mol\right)\) | 17,6 (g) | \(V_{CO_2\left(đkc\right)}=n_{CO_2}.24,79=0,4.24,79=9,916\left(l\right)\) |
| N2 | \(M_{N_2}=2M_N=2.14=28\left(\dfrac{g}{mol}\right)\) | \(n_{N_2}=\dfrac{V_{N_2\left(đkc\right)}}{24,79}=\dfrac{4,958}{24,79}=0,2\left(mol\right)\) | \(m_{N_2}=M_{N_2}.n_{N_2}=28.0,2=5,6\left(g\right)\) | 4,958 (l) |
| H2 | \(M_{H_2}=2M_H=2.1=2\left(\dfrac{g}{mol}\right)\) | 0,5 (mol) | \(m_{H_2}=M_{H_2}.n_{H_2}=2.0,5=1\left(g\right)\) | \(V_{H_2\left(đkc\right)}=n_{H_2}.24,79=0,5.24,79=12,395\left(l\right)\) |