\(n_{Al_2O_3}=\dfrac{10,2}{102}=0,1\left(mol\right)\\ n_{HCl}=\dfrac{7,3}{36,5}=0,2\left(mol\right)\\ pthh:Al_2O_3+6HCl\rightarrow2AlCl_3+3H_2O\\ LTL:\dfrac{0,1}{2}>\dfrac{0,2}{6}\)
=> nhôm oxit dư
\(n_{Al_2O_3\left(p\text{ư}\right)}=\dfrac{1}{6}n_{HCl}=\dfrac{1}{30}\left(mol\right)\\ m_{Al\left(d\right)}=\left(0,1-\dfrac{1}{30}\right).102=6,8\left(g\right)\\ n_{AlCl_3}=\dfrac{1}{3}n_{HCl}=\dfrac{1}{15}\left(mol\right)\\ m_{AlCl_3}=\dfrac{1}{15}.133,5=8,9\left(g\right)\)
a. \(n_{Al_2O_3}=\dfrac{10.2}{102}=0,1\left(mol\right)\)
\(n_{HCl}=\dfrac{7.3}{36,5}=0,2\left(mol\right)\)
PTHH : Al2O3 + 6HCl -> 2AlCl3 + 3H2O
\(\dfrac{0.2}{6}\) 0,2 \(\dfrac{0.4}{6}\)
Ta thấy : \(\dfrac{0.1}{1}>\dfrac{0.2}{6}\) => Al2O3 dư
\(m_{Al_2O_3\left(dư\right)}=\left(0,2-\dfrac{0.2}{6}\right).102=17\left(g\right)\)
b. \(m_{AlCl_3}=\dfrac{0.4}{6}.133,5=8,9\left(g\right)\)
a/
`n_(Al_2O_3) = (10,2)/)(27 . 2 + 16. 3) =0,1 mol`
`n_(HCl) = (7,3)/(1+ 35,5) = 0,2 mol`
PTHH :
`Al_2O_3 + 6HCl -> 2AlCl_3 + 3H_2O`
Nhận thấy : `(0,1)/1 > 0,2/6 -> Al_2O_3` dư và tính theo `HCl`
Ta có :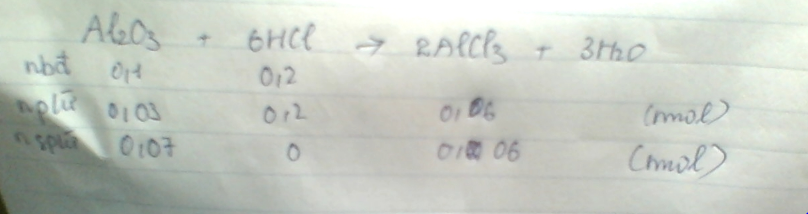
`m_(Al_2O_3)` dư `= 0,07 . 102 =7,14g`
`m_(AlCl_3)=0,06 .133,5 =8,01g`


















