C19:C. Toluen làm mất màu dung dịch KMnO4 khi đun nóng.
C20 : D
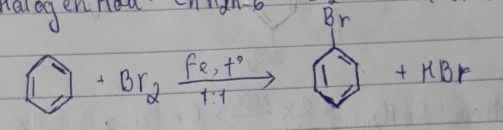
C21: C
CH3OH + Na --> CH3ONa + 1/2H2
0,04 <--- 0,02 (mol)
nH2=0,448/22,4=0,02(mol)
mCH3OH=0,04 . 46 = 1,84(g)
C22:A : propen
C23 : B : etanol
C24:
A đúng
B sai C6H5OH + NaOH → C6H5ONa + H2O
C sai vì Phenol không tác dụng NaHCO3
D sai C6H5OH + Na ---> C6H5ONa + H2
C25 :C
C26:B
\(CH_3CHO\left(andehitexetic\right)+H_2\xrightarrow[xt:Ni]{t^o}C_2H_5OH\left(ancoletylic\right)\)
C27 : A : axit axetic
C28 : B : phenol
Tự luận :
C1:
a) .\(C_6H_5OH+Na\rightarrow C_6H_5ONa+\dfrac{1}{2}H_2\)
b).\(2C_2H_2+5O_2\left(dư\right)\underrightarrow{t^o}2H_2O+4CO_2\)
c). CH\(_2\)= CH\(_2\) + HCl -> CH\(_3\)-CH\(_2\)Cl
d).CH\(_3\)COOH + NaHCO\(_3\) -> CH\(_3\)COONa + CO\(_2\) + H\(_2\)O
C2:
\(nH_2\) \(=\dfrac{0,448}{22,4}=0,02\left(mol\right)\)
a) \(AOH\rightarrow\dfrac{1}{2}H_2\)
0,04<----0,02
\(MA=\dfrac{2,4}{0,04}=60g/mol\)
-> A + 17= 60g/mol
-> MR = 43 g/mol (\(C_3H_7\) )
->CTPT của A là \(C_3H_7OH\)
b).
CH\(_3\)-CH\(_2\)-\(OH\): propan-1-ol
CH\(_3\)-CH(OH)-\(CH_3\) : propan-2-ol






